Abstract
Introduction
Opioids are commonly used as analgesics; however, like any medicine, they can produce adverse drug reactions (ADRs), including nausea, constipation, dependence, and respiratory depression, that result in harmful and fatal events. Therefore, it is essential to monitor the safety of these drugs in clinical practice.
Objective
This study aimed to characterize the safety profile of opioids by conducting a descriptive study based on a spontaneous reporting system (SRS) for ADRs in The Netherlands, focusing on abuse, misuse, medication errors, and differences between sexes.
Methods
Reports submitted to the Netherlands Pharmacovigilance Centre Lareb from January 2003 to December 2021 with an opioid drug as the suspected/interacting medicine were analyzed. Reporting odds ratios (RORs) for drug-ADR combinations were calculated, analyzed, and corrected for sex and drug utilization (expenditure) for the Dutch population.
Results
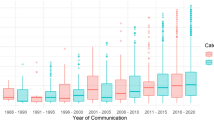
A total of 8769 reports were analyzed. Tramadol was the opioid with the most reports during the period (n = 2746), while oxycodone or tramadol had the highest number of reports per year in the study period. The most reported ADRs from opioid use were nausea, followed by dizziness and vomiting, independent of sex, and all of them were more often reported in women. Vomiting associated with tramadol (ROR females/males = 2.17) was significantly higher in women. Buprenorphine was responsible for most ADRs when corrected for expenditure, with high RORs observed with application site hypersensitivity, application site reaction, and application site rash. Fentanyl gave rise to most of the reports of ADRs concerning abuse, misuse, and medication errors.
Conclusion
Patients treated with opioids experienced ADRs, primarily nausea, dizziness, and vomiting. For those groups of drugs, no significant differences were found between the sexes, except for the vomiting associated with tramadol. In general, ADRs related to opioids presented higher RORs when uncorrected and corrected for sexes and expenditure than other drugs. There was more disproportionate reporting for ADRs concerning abuse, misuse, and medication errors for opioids than other drugs in the Dutch SRS.


Similar content being viewed by others
References
Pathan H, J Williams. Basic opioid pharmacology: an update. Br J Pain. 2012;6(1):11–6.
Bowdle TA. Adverse effects of opioid agonists and agonist-antagonists in anaesthesia. Drug Saf. 1998;19(3):173–89.
Oxycodone KE. J Pain Symptom Manag. 2005;29(5 Suppl):S47-56.
Zöllner C, Stein C. Opioids. Analgesia. 2006:31–63.
National Institute on Drug Abuse. Prescription opioids DrugFacts. 2021. https://nida.nih.gov/publications/drugfacts/prescription-opioids.
Fields HL. The doctor’s dilemma: opiate analgesics and chronic pain. Neuron. 2011;69(4):591–4.
Leppert W, Krajnik M, Wordliczek J. Delivery systems of opioid analgesics for pain relief: a review. Curr Pharm Des. 2013;19(41):7271–93.
Packiasabapathy S, S. Sadhasivam. Gender, genetics, and analgesia: understanding the differences in response to pain relief. J Pain Res. 2018;11:2729–39.
Bartley EJ, Fillingim RB. Sex differences in pain: a brief review of clinical and experimental findings. Br J Anaesth. 2013;111(1):52–8.
Serdarevic M, Striley CW, Cottler LB. Sex differences in prescription opioid use. Curr Opin Psychiatry. 2017;30(4):238–46.
Marie Preciado S, et al. National gender differences in the prescribing of opioid medications from 2006 to 2015. J Opioid Manag. 2020;16(3):197–208.
Campbell CI, et al. Age and gender trends in long-term opioid analgesic use for noncancer pain. Am J Public Health. 2010;100(12):2541–7.
Samulowitz A, et al. “Brave Men” and “Emotional Women”: a theory-guided literature review on gender bias in health care and gendered norms towards patients with chronic pain. Pain Res Manag. 2018;2018:6358624.
Amir C, et al. Test-retest reliability of an adaptive thermal pain calibration procedure in healthy volunteers. J Pain. 2022;23(9):1543–55.
Mogil JS, Chanda ML. The case for the inclusion of female subjects in basic science studies of pain. Pain. 2005;117(1–2):1–5.
United Nations Office on Drugs and Crime. World Drug Report 2019: 35 million people worldwide suffer from drug use disorders while only 1 in 7 people receive treatment. 2019. https://www.unodc.org/unodc/en/frontpage/2019/June/world-drug-report-2019_-35-million-people-worldwide-suffer-from-drug-use-disorders-while-only-1-in-7-people-receive-treatment.html.
World Health Organization. The selection and use of essential medicines: report of the WHO expert committee, 2017 (including the 20th WHO model list of essential medicines and the 6th model list of essential medicines for children). World Health Organization; 2017.
Cragg A, et al. Risk factors for misuse of prescribed opioids: a systematic review and meta-analysis. Ann Emerg Med. 2019;74(5):634–46.
Berterame S, et al. Use of and barriers to access to opioid analgesics: a worldwide, regional, and national study. Lancet. 2016;387(10028):1644–56.
Zin CS, Chen LC, Knaggs RD. Changes in trends and pattern of strong opioid prescribing in primary care. Eur J Pain. 2014;18(9):1343–51.
Ruscitto A, Smith BH, Guthrie B. Changes in opioid and other analgesic use 1995–2010: repeated cross-sectional analysis of dispensed prescribing for a large geographical population in Scotland. Eur J Pain. 2015;19(1):59–66.
Kalkman GA, et al. Trends in use and misuse of opioids in the Netherlands: a retrospective, multi-source database study. Lancet Public Health. 2019;4(10):e498–505.
Bedene A, et al. Opioid prescription patterns and risk factors associated with opioid use in the Netherlands. JAMA Netw Open. 2019;2(8): e1910223.
Degenhardt L, et al. Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: a multistage systematic review. Lancet Glob Health. 2017;5(12):e1192–207.
Benyamin R, et al. Opioid complications and side effects. Pain Physician. 2008;11(2 Suppl):S105–20.
Trescot AM, et al. Opioid pharmacology. Pain Physician. 2008;11(2 Suppl):S133–53.
Inturrisi CE. Clinical pharmacology of opioids for pain. Clin J Pain. 2002;18(4 Suppl):S3-13.
Baldo P, Francescon S, Fornasier G. Pharmacovigilance workflow in Europe and Italy and pharmacovigilance terminology. Int J Clin Pharm. 2018;40(4):748–53.
National Institute on Drug Abuse. Misuse of prescription drugs research report. National Institute of Drug Abuse; 2020. https://nida.nih.gov/download/37630/misuse-prescription-drugs-research-report.pdf?v=add4ee202a1d1f88f8e1fdd2bb83a5ef.
Ferner RE, Easton C, Cox AR. Deaths from medicines: a systematic analysis of coroners’ reports to prevent future deaths. Drug Saf. 2018;41(1):103–10.
Frequently asked questions (FAQ): drug overdose deaths in Europe. European Monitoring Centre for Drugs and Drug Addiction; 2022.
Casati A, Sedefov R, Pfeiffer-Gerschel T. Misuse of medicines in the European Union: a systematic review of the literature. Eur Addict Res. 2012;18(5):228–45.
Giraudon I, et al. Prescription opioid abuse in the UK. Br J Clin Pharmacol. 2013;76(5):823–4.
Shah K, Barker KA. Out-of-hospital medication errors: a 6-year analysis of the national poison data system. Pharmacoepidemiol Drug Saf. 2009;18(11):1080–5.
Lavon O, Ben-Zeev A, Bentur Y. Medication errors outside healthcare facilities: a national poison centre perspective. Basic Clin Pharmacol Toxicol. 2014;114(3):288–92.
Mulac A, et al. Severe and fatal medication errors in hospitals: findings from the Norwegian Incident Reporting System. Eur J Hosp Pharm. 2021;28(e1):e56–61.
Jambrina AM, et al. Detection and prevention of medication errors by the network of sentinel pharmacies in a Southern European Region. J Clin Med. 2022;12(1):194.
Lobaugh LMY, et al. Medication errors in pediatric anesthesia: a report from the wake up safe quality improvement initiative. Anesth Analg. 2017;125(3):936–42.
Wang HW, et al. An experimental investigation of masking in the US FDA adverse event reporting system database. Drug Saf. 2010;33(12):1117–33.
MedDRA. Medical dictionary for regulatory activities. Maintenance and Support Services Organization; 2022.
World Health Organization. WHO Collaborating Centre for Drug Statistics Methodology. ATC/DDD index 2011. World Health Organization; 2011.
WHO Collaborating Centre for Drug Statistics Methodology. N02A opioids. 2023. https://www.whocc.no/atc_ddd_index/?code=n02a.
Nederland, Z., GIPdatabank. Aantal gebruikers 2013–2018 van Pijnstillers, 2019.
Szumilas M. Explaining odds ratios. J Can Acad Child Adolesc Psychiatry. 2010;19(3):227–9.
Schifano F, et al. The e-psychonaut drugs’ psychopharmacology. Curr Opin Pharmacol. 2021;57:165–74.
Schifano F, Chiappini S. Pregabalin: a range of misuse-related unanswered questions. CNS Neurosci Ther. 2019;25(5):659.
European Monitoring Centre for Drugs and Drug Addiction (EMCDDA). European drug report 2022: trends and developments. EMCDDA; 2023.
Lessenger JE, Feinberg SD. Abuse of prescription and over-the-counter medications. J Am Board Fam Med. 2008;21(1):45–54.
Chiappini S, F Schifano. What about “pharming”? Issues regarding the misuse of prescription and over-the-counter drugs. Brain Sci. 2020;10(10):736.
Chiappini S, F. Schifano. What about “pharming”? Issues regarding the misuse of prescription and over-the-counter drugs. MDPI; 2020. p. 736.
Chiappini S, et al. Pharmacovigilance signals of the opioid epidemic over 10 years: data mining methods in the analysis of pharmacovigilance datasets collecting adverse drug reactions (ADRs) reported to EudraVigilance (EV) and the FDA Adverse Event Reporting System (FAERS). Pharmaceuticals. 2022;15(6):675.
Schifano F, et al. Assessing the 2004–2018 fentanyl misusing issues reported to an international range of adverse reporting systems. Front Pharmacol. 2019;10:46.
European Monitoring Centre for Drugs and Drug Addiction (EMCDDA). Opioids: health and social responses. Lisbon: EMCDDA; 2021.
Centers for Disease Control and Prevention. Assessing benefits and harms of opioid therapy. https://www.cdc.gov/drugoverdose/pdf/assessing_benefits_harms_of_opioid_therapy-a.pdf.
Weesie Y, et al. Voorschrijven van opioïden in de huisartsenpraktijk. Utrecht: Netherlands Institute for Health Services Research (NIVEL); 2016.
Højsted J, Sjøgren P. Addiction to opioids in chronic pain patients: a literature review. Eur J Pain. 2007;11(5):490–518.
de Vries ST, et al. Sex differences in adverse drug reactions reported to the National Pharmacovigilance Centre in the Netherlands: an explorative observational study. Br J Clin Pharmacol. 2019;85(7):1507–15.
Holm L, Ekman E, Jorsäter Blomgren K. Influence of age, sex and seriousness on reporting of adverse drug reactions in Sweden. Pharmacoepidemiol Drug Saf. 2017. 26(3):335–43.
Zopf Y, et al. Women encounter ADRs more often than do men. Eur J Clin Pharmacol. 2008;64(10):999–1004.
Tran C, et al. Gender differences in adverse drug reactions. J Clin Pharmacol. 1998;38(11):1003–9.
Lopes GS, et al. Sex differences in type and occurrence of adverse reactions to opioid analgesics: a retrospective cohort study. BMJ Open. 2021;11(6): e044157.
de Vries ST, et al. Sex differences in adverse drug reactions of metformin: a longitudinal survey study. Drug Saf. 2020;43(5):489–95.
Ekhart C, et al. Sex Differences in Reported Adverse Drug Reactions of Selective Serotonin Reuptake Inhibitors. Drug Saf. 2018;41(7):677–83.
Mack KA, et al. Prescription practices involving opioid analgesics among Americans with Medicaid, 2010. J Health Care Poor Underserved. 2015;26(1):182–98.
Frenk SM, KS Porter, Paulozzi L. Prescription opioid analgesic use among adults: United States, 1999–2012. US Department of Health and Human Services, Centers for Disease Control and Prevention; 2015.
Schieber LZ, et al. Variation in adult outpatient opioid prescription dispensing by age and sex—United States, 2008–2018. MMWR Morb Mortal Wkly Rep. 2020;69(11):298–302.
Johnson JL, L Greaves, R Repta. Better science with sex and gender: a primer for health research. Women's Health Res Netw. 2007.
Keefe FJ, et al. Coping with rheumatoid arthritis pain: catastrophizing as a maladaptive strategy. Pain. 1989;37(1):51–6.
Forsythe LP, et al. Race and sex differences in primary appraisals, catastrophizing, and experimental pain outcomes. J Pain. 2011;12(5):563–72.
Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev. 1977;84(2):191.
Jackson T, et al. Gender differences in pain perception: The mediating role of self-efficacy beliefs. Sex Roles. 2002;47:561–8.
Pleym H, et al. Gender differences in drug effects: implications for anesthesiologists. Acta Anaesthesiol Scand. 2003;47(3):241–59.
Berkley, K.J., Sex differences in pain. Behav Brain Sci. 1997. 20(3):371–80 (discussion 435–513).
Craft RM. Sex differences in drug- and non-drug-induced analgesia. Life Sci. 2003;72(24):2675–88.
Inglis JM, et al. Documentation of adverse drug reactions to opioids in an electronic health record. Intern Med J. 2021;51(9):1490–6.
Napp Pharmaceuticals Ltd. OxyNorm 5 mg Hard Capsules SmPC. 1999. https://www.medicines.org.uk/emc/product/3850/smpc#UNDESIRABLE_EFFECTS. 2022.
Bristol Laboratories Ltd. Brimisol PR 100 mg Prolonged-Release Tablets SmPC. 2011. 2021. https://www.medicines.org.uk/emc/product/8550/smpc#UNDESIRABLE_EFFECTS. Cited 11 Jan 2023.
Kyowa Kirin Ltd. Abstral 200 microgram sublingual tablets SmPC. 2008. 2022. https://www.medicines.org.uk/emc/product/10265/smpc#UNDESIRABLE_EFFECTS. Cited 2022.
Ethypharm UK Ltd. Actimorph 20 mg Orodispersible tablets SmPC. 2021. https://www.medicines.org.uk/emc/product/13443/smpc#UNDESIRABLE_EFFECTS.
MedlinePlus. Nitroglycerin Spray. https://medlineplus.gov/druginfo/meds/a615006.html. Cited 12 Oct 2022.
Grunenthal Ltd. Tramacet 37.5 mg/ 325 mg film-coated tablets SmPC. 2003. 2022. https://www.medicines.org.uk/emc/product/6630/smpc#ORIGINAL. Cited 11 Jan 2023.
Yezierski RP. The effects of age on pain sensitivity: preclinical studies. Pain Med. 2012;13(Suppl 2):S27–36.
Lautenbacher S, et al. Age changes in pain perception: a systematic-review and meta-analysis of age effects on pain and tolerance thresholds. Neurosci Biobehav Rev. 2017;75:104–13.
Fayaz A, et al. Prevalence of chronic pain in the UK: a systematic review and meta-analysis of population studies. BMJ Open. 2016;6(6): e010364.
Yancik R. Population aging and cancer: a cross-national concern. Cancer J. 2005;11(6):437–41.
Wilder-Smith OHG. Opioid use in the elderly. Eur J Pain. 2005;9(2):137–40.
Chiappini S, et al. Opioid painkiller dependence in a sample of elderly medical inpatients. Psychogeriatrics. 2021;21(3):265–71.
Olthoff MV, et al. Overgevoeligheid voor hulpstoffen in pleisters. Nederlands Tijdschrift voor Geneeskunde; 2019. p. 163.
Alqahtani MS, et al. Advances in oral drug delivery. Front Pharmacol. 2021;12: 618411.
Vander Hulst K, et al. Allergic contact dermatitis from transdermal buprenorphine. Contact Dermat. 2008;59(6):366–9.
Romita P, et al. Contact dermatitis due to transdermal therapeutic systems: a clinical update. Acta Biomed. 2018;90(1):5–10.
Chiappini S., et al., Pharmacovigilance signals of the opioid epidemic over 10 years: data mining methods in the analysis of pharmacovigilance datasets collecting adverse drug reactions (ADRs) Reported to EudraVigilance (EV) and the FDA Adverse Event Reporting System (FAERS). Pharmaceuticals (Basel). 2022;15(6).
Häkkinen M, Vuori E, Ojanperä I. Prescription opioid abuse based on representative postmortem toxicology. Forensic Sci Int. 2014;245:121–5.
Comer SD, Cahill CM. Fentanyl: Receptor pharmacology, abuse potential, and implications for treatment. Neurosci Biobehav Rev. 2019;106:49–57.
O’Connor AB. Is actiq use in noncancer-related pain really “a recipe for success”? Pain Med. 2008;9(2):258–60.
Passik SD, Kirsh KL. Weighing in on the off-label use of Actiq™ for noncancer-related pain: a recipe for success or a recipe for disaster? Pain Med. 2007;8(2):130–3.
Carreyrou J. Narcotic ‘lollipop’is big seller despite FDA curbs. Wall Street J; 2006:735399–114.
European Medicines Agency. Withdrawal Assessment report: Effentora. Amsterdam: European Medicines Agency; 2013.
European Medicines Agency. Questions and answers, Withdrawal of the application for a change to the marketing authorisation for Effentora (fentanyl). Amsterdam: European Medicines Agency; 2013.
Takeda Pharma A/S. Instanyl 50 micrograms/dose nasal spray, solution SmPC. 2009. https://www.ema.europa.eu/en/documents/product-information/instanyl-epar-product-information_en.pdf.
Teva B.V. Effentora 100 micrograms buccal tablets. 2008. https://www.ema.europa.eu/en/documents/product-information/effentora-epar-product-information_en.pdf.
Jain P, et al. Evaluation of opioid overdose reports in patients treated with extended-release naltrexone: postmarketing data from 2006 to 2018. Drug Saf. 2021;44(3):351–9.
Ekhart C, de Vries T, van Hunsel F. Psychiatric adverse drug reactions in the paediatric population. Arch Dis Child. 2020;105(8):749–55.
Hazell L, Shakir SA. Under-reporting of adverse drug reactions : a systematic review. Drug Saf. 2006;29(5):385–96.
Coleman JJ, Pontefract SK. Adverse drug reactions. Clin Med (Lond). 2016;16(5):481–5.
Lewer D, et al. Causes of death among people who used illicit opioids in England, 2001–18: a matched cohort study. Lancet Public Health. 2022;7(2):e126–35.
Güner MD, Ekmekci PE. Healthcare professionals’ pharmacovigilance knowledge and adverse drug reaction reporting behavior and factors determining the reporting rates. J Drug Assess. 2019;8(1):13–20.
Cheema E, et al. Barriers to reporting of adverse drugs reactions: a cross sectional study among community pharmacists in United Kingdom. Pharm Pract (Granada). 2017;15(3):931.
Joaquim J, et al. All-round approaches to increase adverse drug reaction reports: a scoping review. Drugs & Therapy Perspectives. 2023;39:249–61.
Matos C, Rodrigues L, Joaquim J. Attitudes and opinions of Portuguese community pharmacy professionals towards patient reporting of adverse drug reactions and the pharmacovigilance system. Drugs Ther Perspect. 2017;33:188–94.
Pande S. Causality or relatedness assessment in adverse drug reaction and its relevance in dermatology. Indian J Dermatol. 2018;63(1):18–21.
Wallerstedt SM, G Brunlöf, A. Sundström. Rates of spontaneous reports of adverse drug reactions for drugs reported in children. Drug Saf. 2011;34(8):669–82.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this study.
Conflict of interest
Moa Gustaffson, Cristiano Matos, João Joaquim, Joep Scholl and Florence van Hunsel have no conflicts of interest to declare that are directly relevant to the contents of this study.
Ethical approval
Ethical approval was not needed for this study.
Consent to participate
No approval or consent was needed for this study.
Consent for publication
No approval or consent was needed for this study.
Availability of data and materials
The datasets for this manuscript are not publicly available due to the data protection policy of the Pharmacovigilance Centre Lareb. Requests to access the datasets should be directed to the first author and will be granted on reasonable request.
Code availability
The SQL statements for the data used in this article are not publicly available due to the data protection policy of Lareb. Requests to access the datasets should be directed to the first author and will be granted on reasonable request.
Authors’ contributions
The original study protocol was designed by MG and CM with input from FvH. JS established the query and dataset. Data analysis was performed by MG and CM, and the design of the manuscript was determined by all authors. All authors contributed to the final data analysis and manuscript drafting and revision. All authors approved the final version to be published and agreed to be accountable for all aspects of the work.
Privacy statement
All personal data from the patient’s reporting form is handled in accordance with the General Data Protection Regulation (GDPR) and the general privacy regulation of the Pharmacovigilance Centre Lareb.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gustafsson, M., Matos, C., Joaquim, J. et al. Adverse Drug Reactions to Opioids: A Study in a National Pharmacovigilance Database. Drug Saf 46, 1133–1148 (2023). https://doi.org/10.1007/s40264-023-01351-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-023-01351-y