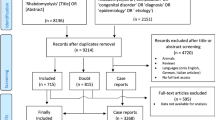
Abstract
Objective
To analyze the clinical characteristics, incidence, and distribution of drug-associated muscle adverse reactions (DAMAR) in real-world inpatients, to provide valuable references for clinical medication use.
Methods
We conducted an automatic retrospective monitoring of inpatients from May 1, 2022, to April 30, 2023, to collect information on adverse drug reactions (ADR) of patients and conducted subsequent analyses.
Results
Among 102,430 hospitalizations, 1106 cases of DAMARs were identified, yielding an incidence of 1.08%, including 125 cases of rhabdomyolysis at an incidence of 0.12%. Seventy-five percent of the patients experienced muscle adverse reactions within 5 days after taking medication, with a median elevated creatine kinase (CK) value of 420.4 IU/L. Risk factors of DAMAR include age ≥ 65, male sex, obesity, hypertension, hepatic and renal insufficiency, and anemia. No significant correlation was observed between the duration of surgery and CK elevation, while the surgical procedure itself had an impact. The 114 drugs associated were predominantly nervous system drugs, anti-infectives for systemic use, and cardiovascular system drugs, with levofloxacin, pregabalin, and parecoxib being the most frequently associated drugs.
Conclusion
Healthcare professionals should be vigilant with patients exhibiting the identified risk factors. Monitoring creatine kinase and related indices when using myotoxic drugs is crucial to preventing serious adverse reactions, ultimately preserving patients’ quality of life.





Similar content being viewed by others
Data availability
The data used to support the findings of this study are available upon reasonable request from the authors.
References
Rosenson RS, Baker SK, Jacobson TA et al (2014) An assessment by the statin muscle safety task force: 2014 update [J]. J Clin Lipidol 8(3 Suppl):S58-71
Kodadek L, Carmichael II SP, Seshadri A et al (2022) Rhabdomyolysis: an American Association for the surgery of trauma critical care committee clinical consensus document [J]. Trauma Surg Acute Care Open 7(1):e000836
McMahon GM, Zeng X, Waikar SS (2013) A risk prediction score for kidney failure or mortality in rhabdomyolysis [J]. JAMA Intern Med 173(19):1821–8
Newman CB, Preiss D, Tobert JA et al (2019) Statin safety and associated adverse events: a scientific statement from the American Heart Association [J]. Arterioscler Thromb Vasc Biol 39(2):e38–e81
Kellick KA, Bottorff M, Toth PP et al (2014) A clinician’s guide to statin drug-drug interactions [J]. Journal of clinical lipidology 8(3 Suppl):S30-46
Janssen L, Allard NAE, Saris CGJ et al (2020) Muscle toxicity of drugs: when drugs turn physiology into pathophysiology [J]. Physiol Rev 100(2):633–72
Zhao A, Guo D, Zhu M et al (2023) Analysis of 1 702 cases of drug-associated muscle adverse reactions [J]. Chin J Clin Pharmacol 39(16):2393–6
Zhao A, Guo D, Zhu M et al (2023) Establishment and optimization of a module for automatic monitoring drug-associated muscle adverse reactions based on HIS [J]. Chin J Drug Appl Mon 20(3):176-9, 210
Yao C, Liu D, Guo D et al (2020) The development on active surveillance and assessment system-II of adverse drug events [J]. Chin J Drug Appl Mon 17(06):387–91
Kyriakides T, Angelini C, Schaefer J et al (2010) EFNS guidelines on the diagnostic approach to pauci- or asymptomatic hyperCKemia [J]. Eur J Neurol 17(6):767–73
Gabow PA, Kaehny WD, Kelleher SP (1982) The spectrum of rhabdomyolysis [J]. Medicine 61(3):141–52
Naranjo CA, Busto U, Sellers EM et al (1981) A method for estimating the probability of adverse drug reactions [J]. Clin Pharmacol Ther 30(2):239–45
Dai-Hong G, Cheng-Xuan Y (2021) Expert consensus on automated surveillance and evaluation of clinical medication risk based on hospital information system data [J]. Chin J Drug Appl Mon 18(05):277–87
Guo H, Li P, Guo D, Gao A, Zhao P, Fu A, Li C, Lu J (2023) Analysis of clinical characteristics and automatic monitoring of drug-induced arrhythmias in 167,546 inpatients. Eur J Clin Pharmacol. 79(6):759–765
Kong X, Guo D, Liu S, Zhu Y, Yu C (2021) Incidence, characteristics and risk factors for drug-induced liver injury in hospitalized patients: a matched case-control study. Br J Clin Pharmacol. 87(11):4304–4312
Dugué A, Bagheri H, Lapeyre-Mestre M et al (2004) Detection and incidence of muscular adverse drug reactions: a prospective analysis from laboratory signals [J]. Eur J Clin Pharmacol 60(4):285-92
Ostrowski P, Bonczar M, Avram AE et al (2023) Safety monitoring of drug-induced muscle injury and rhabdomyolysis: a biomarker-guided approach for clinical practice and drug trials [J]. Clin Chem Lab Med 61(10):1688–99
Gao Z, Liang Y, Wu Z et al (2023) Prevalence of rhabdomyolysis following bariatric surgery and its associated risk factors: a meta-analysis [J]. Obes Surg 33(4):990–1003
Lagandré S, Arnalsteen L, Vallet B et al (2006) Predictive factors for rhabdomyolysis after bariatric surgery [J]. Obes Surg 16(10):1365-70
Melli G, Chaudhry V, Cornblath DR (2005) Rhabdomyolysis: an evaluation of 475 hospitalized patients [J]. Medicine 84(6):377–85
Grigorian A, Gabriel V, Nguyen NT et al (2020) Black race and body mass index are risk factors for rhabdomyolysis and acute kidney injury in trauma [J]. J Invest Surg 33(3):283–90
Pedro-Botet J, Millán Núñez-Cortés J, Chillarón JJ et al (2016) Severity of statin-induced adverse effects on muscle and associated conditions: data from the DAMA study [J]. Expert Opin Drug Saf 15(12):1583-7
Jones JD, Kirsch HL, Wortmann RL et al (2014) The causes of drug-induced muscle toxicity [J]. Curr Opin Rheumatol 26(6):697–703
Conforti A, Chiamulera C, Moretti U et al (2007) Musculoskeletal adverse drug reactions: a review of literature and data from ADR spontaneous reporting databases [J]. Curr Drug Saf 2(1):47–63
Carroll MW, Choi H, Min S et al (2012) Rhabdomyolysis in a patient treated with linezolid for extensively drug-resistant tuberculosis [J]. Clin Infect Dis 54(11):1624–7
Vinci P, Panizon E, Tosoni LM et al (2021) Statin-associated myopathy: emphasis on mechanisms and targeted therapy [J]. Int J Mol Sci 22(21)
Ochs-Balcom HM, Nguyen LM, Ma C et al (2019) Clinical features related to statin-associated muscle symptoms [J]. Muscle Nerve 59(5):537–43
Warden BA, Guyton JR, Kovacs AC et al (2023) Assessment and management of statin-associated muscle symptoms (SAMS): a clinical perspective from the National Lipid Association [J]. J Clin Lipidol 17(1):19–39
Lu H, Cole SR, Howe CJ et al (2022) Toward a clearer definition of selection bias when estimating causal effects [J]. Epidemiology (Cambridge, Mass) 33(5):699–706
Funding
This study was supported by the Key Project of Medical Innovation Project in 2017 (No. 17CXZ010) and “the Project of Monitoring and Evaluation of the Use of Key Clinical Drugs” commissioned by the Chinese Association of Research Hospitals (No. Y2023FH-YWPJ03). The funding body had no role in this study.
Author information
Authors and Affiliations
Contributions
AZ: conception, design, data collection, analysis of data, drafting, and revision of manuscript. DG: conception, design, interpretation of results, and critical revision of manuscript. MZ and AG: interpretation of results revision of manuscript. PL and AF: revision of manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethical approval
The study was approved by the Medical Ethics Committee of PLA General Hospital (No. S2018-54-01). This consent protocol was reviewed, and the need for written and informed consent was waived by the Medical Ethics Committee of PLA General Hospital. All patient data were kept strictly confidential.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, A., Guo, D., Zhu, M. et al. Incidence, characteristics, and risk factors of drug-associated muscle adverse reaction: a retrospective real-world study of inpatients. Eur J Clin Pharmacol 80, 911–918 (2024). https://doi.org/10.1007/s00228-024-03662-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-024-03662-0