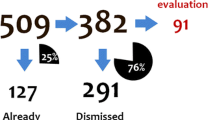
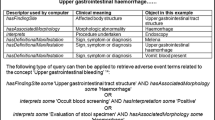
Abstract
Introduction
Pharmacovigilance programs protect patient health and safety by identifying adverse event signals through postmarketing surveillance of claims data and spontaneous reports. Electronic health records (EHRs) provide new opportunities to address limitations of traditional approaches and promote discovery-oriented pharmacovigilance.
Methods
To evaluate the current state of EHR-based medication safety signal identification, we conducted a scoping literature review of studies aimed at identifying safety signals from routinely collected patient-level EHR data. We extracted information on study design, EHR data elements utilized, analytic methods employed, drugs and outcomes evaluated, and key statistical and data analysis choices.
Results
We identified 81 eligible studies. Disproportionality methods were the predominant analytic approach, followed by data mining and regression. Variability in study design makes direct comparisons difficult. Studies varied widely in terms of data, confounding adjustment, and statistical considerations.
Conclusion
Despite broad interest in utilizing EHRs for safety signal identification, current efforts fail to leverage the full breadth and depth of available data or to rigorously control for confounding. The development of best practices and application of common data models would promote the expansion of EHR-based pharmacovigilance.



Similar content being viewed by others
References
World Health Organization. What is pharmacovigilance? [cited 22 Jul 2022]. Available at: https://www.who.int/teams/regulation-prequalification/regulation-and-safety/pharmacovigilance.
Hauben M, Aronson JK. Defining “signal” and its subtypes in pharmacovigilance based on a systematic review of previous definitions. Drug Saf. 2009;32:99–110.
Coloma PM, Trifiro G, Patadia V, Sturkenboom M. Postmarketing safety surveillance: where does signal detection using electronic healthcare records fit into the big picture? Drug Saf. 2013;36:183–97.
US Food and Drug Administration. Questions and answers on FDA’s adverse event reporting system (FAERS). 2018. Available at: https://www.fda.gov/drugs/surveillance/questions-and-answers-fdas-adverse-event-reporting-system-faers.
European Medicines Agency. EudraVigilance system overview [cited 25 Aug 2022]. Available at: https://www.ema.europa.eu/en/human-regulatory/research-development/pharmacovigilance/eudravigilance/eudravigilance-system-overview.
World Health Organization, Uppsala Monitoring Centre. What is VigiBase? [cited 25 Aug 2022]. Available at: https://who-umc.org/vigibase/.
Desai RJ, Matheny ME, Johnson K, Marsolo K, Curtis LH, Nelson JC, et al. Broadening the reach of the FDA Sentinel system: a roadmap for integrating electronic health record data in a causal analysis framework. NPJ Digit Med. 2021;4:170.
Platt R, Brown JS, Robb M, McClellan M, Ball R, Nguyen MD, et al. The FDA sentinel initiative—an evolving national resource. N Engl J Med. 2018;379:2091–3.
European Medicines Agency. Data analysis and real world interrogation network (DARWIN EU) [cited 14 May 2023]. Available at: https://www.darwin-eu.org.
National Institutes of Health. All of us research program [cited 13 May 2023]. Available at: https://allofus.nih.gov.
European Health Data and Evidence Network [cited 13 May 2023]. Available at: https://www.ehden.eu.
Vilar S, Friedman C, Hripcsak G. Detection of drug–drug interactions through data mining studies using clinical sources, scientific literature and social media. Brief Bioinform. 2018;19:863–77.
Wilson AM, Thabane L, Holbrook A. Application of data mining techniques in pharmacovigilance. Br J Clin Pharmacol. 2004;57:127–34.
Almenoff JS, Pattishall EN, Gibbs TG, DuMouchel W, Evans SJW, Yuen N. Novel statistical tools for monitoring the safety of marketed drugs. Clin Pharmacol Ther. 2007;82:157–66.
Lu Z. Information technology in pharmacovigilance: benefits, challenges, and future directions from industry perspectives. Drug Healthc Patient Saf. 2009;1:35–45.
Ho T-B, Le L, Thai DT, Taewijit S. Data-driven approach to detect and predict adverse drug reactions. Curr Pharm Des. 2016;22:3498–526.
Bates DW, Evans RS, Murff H, Stetson PD, Pizziferri L, Hripcsak G. Detecting adverse events using information technology. J Am Med Inform Assoc JAMIA. 2003;10:115–28.
McMahon AW, Wharton GT, Bonnel R, DeCelle M, Swank K, Testoni D, et al. Pediatric post-marketing safety systems in North America: assessment of the current status. Pharmacoepidemiol Drug Saf. 2015;24:785–92.
Izem R, Sanchez-Kam M, Ma H, Zink R, Zhao Y. Sources of safety data and statistical strategies for design and analysis: postmarket surveillance. Ther Innov Regul Sci. 2018;52:159–69.
Wong A, Plasek JM, Montecalvo SP, Zhou L. Natural language processing and its implications for the future of medication safety: a narrative review of recent advances and challenges. Pharmacotherapy. 2018;38:822–41.
Zarrinpar A, David Cheng T-Y, Huo Z. What can we learn about drug safety and other effects in the era of electronic health records and big data that we would not be able to learn from classic epidemiology? J Surg Res. 2020;246:599–604.
Kalinin AA, Higgins GA, Reamaroon N, Soroushmehr S, Allyn-Feuer A, Dinov ID, et al. Deep learning in pharmacogenomics: from gene regulation to patient stratification. Pharmacogenomics. 2018;19:629–50.
Warrer P, Hansen EH, Juhl-Jensen L, Aagaard L. Using text-mining techniques in electronic patient records to identify ADRs from medicine use. Br J Clin Pharmacol. 2012;73:674–84.
Luo Y, Thompson WK, Herr TM, Zeng Z, Berendsen MA, Jonnalagadda SR, et al. Natural language processing for EHR-based pharmacovigilance: a structured review. Drug Saf. 2017;40:1075–89.
Moore TJ, Furberg CD. Electronic health data for postmarket surveillance: a vision not realized. Drug Saf. 2015;38:601–10.
Wisniewski AFZ, Bate A, Bousquet C, Brueckner A, Candore G, Juhlin K, et al. Good signal detection practices: evidence from IMI PROTECT. Drug Saf. 2016;39:469–90.
Lee VC. Big data and pharmacovigilance: data mining for adverse drug events and interactions. P T. 2018;43:340–51.
Harpaz R, Callahan A, Tamang S, Low Y, Odgers D, Finlayson S, et al. Text mining for adverse drug events: the promise, challenges, and state of the art. Drug Saf. 2014;37:777–90.
The Health Improvement Network [cited 25 Aug 2022]. Available at: https://www.the-health-improvement-network.com.
García Rodríguez LA, Pérez GS. Use of the UK general practice research database for pharmacoepidemiology. Br J Clin Pharmacol. 1998;45:419–25.
Ryan PB, Stang PE, Overhage JM, Suchard MA, Hartzema AG, DuMouchel W, et al. A comparison of the empirical performance of methods for a risk identification system. Drug Saf. 2013;36(Suppl 1):S143-158.
Chazard E, Ficheur G, Merlin B, Genin M, Preda C, PSIP Consortium, et al. Detection of adverse drug events detection: data aggregation and data mining. Stud Health Technol Inform. 2009;148:75–84.
Edwards RI. Advanced methods in pharmacovigilance and toxicosurveillance. Clin Toxicol. 2009;47:485.
Ryan PB, Powell GE, Pattishall EN, Beach KJ. Performance of screening multiple observational databases for active drug safety surveillance. Pharmacoepidemiol Drug Saf PDS. 2009;18:S78.
Wang X, Hripcsak G, Markatou M, Friedman C. Active computerized pharmacovigilance using natural language processing, statistics, and electronic health records: a feasibility study. J Am Med Inform Assoc. 2009;16:328–37.
Brownstein JS, Murphy SN, Goldfine AB, Grant RW, Sordo M, Gainer V, et al. Rapid identification of myocardial infarction risk associated with diabetes medications using electronic medical records. Diabetes Care. 2010;33:526–31.
Harpaz R, Haerian K, Chase HS, Friedman C. Mining electronic health records for adverse drug effects using regression based methods. In: Proceedings of the 1st ACM International Health Informatics Symposium—IHI 10. Arlington: ACM Press; 2010 [cited 22 Jul 2022]. p. 100. Available at: http://portal.acm.org/citation.cfm?doid=1882992.1883008.
Brown JS, Dashevsky I, Fireman B, Herrinton L, McClure D, Murphy M, et al. Data mining with a tree-based scan statistic. Pharmacoepidemiol Drug Saf. 2011;20:S331.
Chazard E, Ficheur G, Bernonville S, Luyckx M, Beuscart R. Data mining to generate adverse drug events detection rules. IEEE Trans Inf Technol Biomed Publ IEEE Eng Med Biol Soc. 2011;15:823–30.
Coloma PM, Schuemie MJ, Trifirò G, Gini R, Herings R, Hippisley-Cox J, et al. Combining electronic healthcare databases in Europe to allow for large-scale drug safety monitoring: the EU-ADR Project. Pharmacoepidemiol Drug Saf. 2011;20:1–11.
Coloma PM, Trifiro G, Gini R, Herings R, Mazzaglia G, Giaquinto C, et al. Comparison of methods for drug safety signal detection using electronic healthcare record (EHR) databases: the added value of longitudinal, time-stamped patient information. Pharmacoepidemiol Drug Saf. 2011;20:S142.
Ferrajolo C, Trifiro G, Coloma PM, Schuemie MJ, Gini R, Herings R, et al. Drug use and acute liver injury in children: signal detection using multiple healthcare databases. Drug Saf. 2011;34:983–4.
Ji Y, Ying H, Dews P, Mansour A, Tran J, Miller RE, et al. A potential causal association mining algorithm for screening adverse drug reactions in postmarketing surveillance. IEEE Trans Inf Technol Biomed Publ IEEE Eng Med Biol Soc. 2011;15:428–37.
Park MY, Yoon D, Lee K, Kang SY, Park I, Lee S-H, et al. A novel algorithm for detection of adverse drug reaction signals using a hospital electronic medical record database. Pharmacoepidemiol Drug Saf. 2011;20:598–607.
Trifiro G, Patadia V, Schuemie MJ, Coloma PM, Gini R, Herings R, et al. EU-ADR healthcare database network vs. spontaneous reporting system database: preliminary comparison of signal detection. Stud Health Technol Inform. 2011;166:25–30.
LePendu P, Bauer-Mehren A, Iyer S, Shah NH. Abstract 15727: Analyzing unstructured clinical notes for phase IV drug safety surveillance. Circulation. 2012;126(suppl_21):A15727.
Star K, Strandell J, Friden S, Sallstedt L, Johansson J, Edwards RI. Temporal pattern discovery on electronic health records—a source of reference in signal detectionwork. Pharmacoepidemiol Drug Saf. 2012;21:347.
Yoon D, Park MY, Choi NK, Park BJ, Kim JH, Park RW. Detection of adverse drug reaction signals using an electronic health records database: comparison of the Laboratory Extreme Abnormality Ratio (CLEAR) algorithm. Clin Pharmacol Ther. 2012;91:467–74.
Afzal Z, Kors JA, Sturkenboom MC, Schuemie MJ. Identifying drug-safety signals in electronic health records: an evaluation of automated case-detection algorithms with different sensitivity and specificity. Pharmacoepidemiol Drug Saf. 2013;22:285–6.
An L, Ravindran PP, Renukunta S, Denduluri S. Co-medication of pravastatin and paroxetine-a categorical study. J Clin Pharmacol. 2013;53:1212–9.
Harpaz R, Vilar S, DuMouchel W, Salmasian H, Haerian K, Shah NH, et al. Combing signals from spontaneous reports and electronic health records for detection of adverse drug reactions. J Am Med Inform Assoc. 2013;20:413–9.
Kulldorff M, Dashevsky I, Avery TR, Chan AK, Davis RL, Graham D, et al. Drug safety data mining with a tree-based scan statistic. Pharmacoepidemiol Drug Saf. 2013;22:517–23.
Lependu P, Iyer SV, Bauer-Mehren A, Harpaz R, Ghebremariam YT, Cooke JP, et al. Pharmacovigilance using clinical text. AMIA Jt Summits Transl Sci Proc AMIA Jt Summits Transl Sci. 2013;2013:109.
LePendu P, Iyer SV, Bauer-Mehren A, Harpaz R, Mortensen JM, Podchiyska T, et al. Pharmacovigilance using clinical notes. Clin Pharmacol Ther. 2013;93:547–55.
Lian Duan L, Khoshneshin M, Street WN, Liu M. Adverse drug effect detection. IEEE J Biomed Health Inform. 2013;17:305–11.
Liu M, McPeek Hinz ER, Matheny ME, Denny JC, Schildcrout JS, Miller RA, et al. Comparative analysis of pharmacovigilance methods in the detection of adverse drug reactions using electronic medical records. J Am Med Inform Assoc JAMIA. 2013;20:420–6.
Reps JM, Garibaldi JM, Aickelin U, Soria D, Gibson J, Hubbard R. Comparison of algorithms that detect drug side effects using electronic healthcare databases. Soft Comput. 2013;17:2381–97.
Sauzet O, Carvajal A, Escudero A, Molokhia M, Cornelius VR. Illustration of the Weibull shape parameter signal detection tool using electronic healthcare record data. Drug Saf. 2013;36:995–1006.
Eriksson R, Werge T, Jensen LJ, Brunak S. Dose-specific adverse drug reaction identification in electronic patient records: temporal data mining in an inpatient psychiatric population. Drug Saf. 2014;37:237–47.
Ferrajolo C, Coloma PM, Verhamme KMC, Schuemie MJ, de Bie S, Gini R, et al. Signal detection of potentially drug-induced acute liver injury in children using a multi-country healthcare database network. Drug Saf. 2014;37:99–108.
Iyer SV, Harpaz R, LePendu P, Bauer-Mehren A, Shah NH. Mining clinical text for signals of adverse drug–drug interactions. J Am Med Inform Assoc JAMIA. 2014;21:353–62.
Ji Y, Ying H, Tran J, Dews P, Mansour A, Massanari RM. A temporal interestingness measure for drug interaction signal detection in post-marketing surveillance. Annu Int Conf IEEE Eng Med Biol Soc IEEE Eng Med Biol Soc Annu Int Conf. 2014;2014:2722–5.
Li Y, Salmasian H, Vilar S, Chase H, Friedman C, Wei Y. A method for controlling complex confounding effects in the detection of adverse drug reactions using electronic health records. J Am Med Inform Assoc. 2014;21:308–14.
Patel VN, Kaelber DC. Using aggregated, de-identified electronic health record data for multivariate pharmacosurveillance: a case study of azathioprine. J Biomed Inform. 2014;52:36–42.
Roitmann E, Eriksson R, Brunak S. Patient stratification and identification of adverse event correlations in the space of 1190 drug related adverse events. Front Physiol. 2014;5:Article 332.
Cederholm S, Hill G, Asiimwe A, Bate A, Bhayat F, Persson Brobert G, et al. Structured assessment for prospective identification of safety signals in electronic medical records: evaluation in the health improvement network. Drug Saf. 2015;38:87–100.
Du L, Chakraborty A, Chiang C-W, Cheng L, Quinney SK, Wu H, et al. Graphic mining of high-order drug interactions and their directional effects on myopathy using electronic medical records. CPT Pharmacomet Syst Pharmacol. 2015;4:481–8.
Girardeau Y, Trivin C, Durieux P, Le Beller C, Louet Agnes L-L, Neuraz A, et al. Detection of drug–drug interactions inducing acute kidney injury by electronic health records mining. Drug Saf. 2015;38:799–809.
Li Y, Ryan PB, Wei Y, Friedman C. A method to combine signals from spontaneous reporting systems and observational healthcare data to detect adverse drug reactions. Drug Saf. 2015;38:895–908.
Pacurariu AC, Straus SM, Trifiro G, Schuemie MJ, Gini R, Herings R, et al. Useful interplay between spontaneous ADR reports and electronic healthcare records in signal detection. Drug Saf. 2015;38:1201–10.
Patadia VK, Schuemie MJ, Coloma P, Herings R, van der Lei J, Straus S, et al. Evaluating performance of electronic healthcare records and spontaneous reporting data in drug safety signal detection. Int J Clin Pharm. 2015;37:94–104.
Reps JM, Garibaldi JM, Aickelin U, Gibson JE, Hubbard RB. A supervised adverse drug reaction signalling framework imitating Bradford Hill’s causality considerations. J Biomed Inform. 2015;56:356–68.
Star K, Watson S, Sandberg L, Johansson J, Edwards IR. Longitudinal medical records as a complement to routine drug safety signal analysis. Pharmacoepidemiol Drug Saf. 2015;24:486–94.
Wang G, Jung K, Winnenburg R, Shah NHA. A method for systematic discovery of adverse drug events from clinical notes. J Am Med Inform Assoc. 2015;22:1196–204.
Zhang P, Du L, Wang L, Liu M, Cheng L, Chiang C-W, et al. A mixture dose-response model for identifying high-dimensional drug interaction effects on myopathy using electronic medical record databases. CPT Pharmacomet Syst Pharmacol. 2015;4:474–80.
Hauben M, Liu Q, Hung E, Blackwell W, Fram D, Bate A. Signal detection using temporal pattern discovery (TPD) in electronic health records (EHRs)—lessons from statins and rhabdomyolysis. Pharmacoepidemiol Drug Saf. 2016;25:441–2.
Lorberbaum T, Sampson KJ, Chang JB, Iyer V, Woosley RL, Kass RS, et al. Coupling data mining and laboratory experiments to discover drug interactions causing QT prolongation. J Am Coll Cardiol. 2016;68:1756–64.
Lorberbaum T, Sampson KJ, Woosley RL, Kass RS, Tatonetti NP. An Integrative data science pipeline to identify novel drug interactions that prolong the QT interval. Drug Saf. 2016;39:433–41.
Boland MR, Polubriaginof F, Tatonetti NP. Development of a machine learning algorithm to classify drugs of unknown fetal effect. Sci Rep. 2017;7:12839.
Fan Y, Adam TJ, McEwan R, Pakhomov SV, Melton GB, Zhang R. Detecting signals of interactions between warfarin and dietary supplements in electronic health records. Stud Health Technol Inform. 2017;245:370–4.
Lee S, Choi J, Kim H-S, Kim GJ, Lee KH, Park CH, et al. Standard-based comprehensive detection of adverse drug reaction signals from nursing statements and laboratory results in electronic health records. J Am Med Inform Assoc. 2017;24:697–708.
Personeni G, Bresso E, Devignes M-D, Dumontier M, Smail-Tabbone M, Coulet A. Discovering associations between adverse drug events using pattern structures and ontologies. J Biomed Semant. 2017;8:29.
Wang L, Rastegar-Mojarad M, Liu S, Zhang H, Liu H. Discovering adverse drug events combining spontaneous reports with electronic medical records: a case study of conventional DMARDs and biologics for rheumatoid arthritis. AMIA Jt Summits Transl Sci Proc AMIA Jt Summits Transl Sci. 2017;2017:95–103.
Chen W, Yang J, Wang H-L, Shi Y-F, Tang H, Li G-H. Discovering associations of adverse events with pharmacotherapy in patients with non-small cell lung cancer using modified apriori algorithm. BioMed Res Int. 2018;2018:1245616.
Choi L, Carroll RJ, Beck C, Mosley JD, Roden DM, Denny JC, et al. Evaluating statistical approaches to leverage large clinical datasets for uncovering therapeutic and adverse medication effects. Bioinforma Oxf Engl. 2018;34:2988–96.
Jeong E, Park N, Choi Y, Park RW, Yoon D. Machine learning model combining features from algorithms with different analytical methodologies to detect laboratory-event-related adverse drug reaction signals. PLoS ONE. 2018;13: e0207749.
Patadia VK, Schuemie MJ, Coloma PM, Herings R, van der Lei J, Sturkenboom M, et al. Can electronic health records databases complement spontaneous reporting system databases? A historical-reconstruction of the association of rofecoxib and acute myocardial infarction. Front Pharmacol. 2018;9:594.
Shimai Y, Takeda T, Okada K, Manabe S, Teramoto K, Mihara N, et al. Screening of anticancer drugs to detect drug-induced interstitial pneumonia using the accumulated data in the electronic medical record. Pharmacol Res Perspect. 2018;6: e00421.
Tham MY, Ye Q, Ang PS, Fan LY, Yoon D, Park RW, et al. Application and optimisation of the Comparison on Extreme Laboratory Tests (CERT) algorithm for detection of adverse drug reactions: transferability across national boundaries. Pharmacoepidemiol Drug Saf. 2018;27:87–94.
Vajravelu RK, Scott FI, Mamtani R, Li H, Moore JH, Lewis JD. Medication class enrichment analysis: a novel algorithm to analyze multiple pharmacologic exposures simultaneously using electronic health record data. J Am Med Inform Assoc JAMIA. 2018;25:780–9.
Wang L, Rastegar-Mojarad M, Ji Z, Liu S, Liu K, Moon S, et al. Detecting pharmacovigilance signals combining electronic medical records with spontaneous reports: a case study of conventional disease-modifying antirheumatic drugs for rheumatoid arthritis. Front Pharmacol. 2018;9:875.
Wang X, Zhang P, Chiang C-W, Wu H, Shen L, Ning X, et al. Mixture drug-count response model for the high-dimensional drug combinatory effect on myopathy. Stat Med. 2018;37:673–86.
Whalen E, Hauben M, Bate A. Time series disturbance detection for hypothesis-free signal detection in longitudinal observational databases. Drug Saf. 2018;41:565–77.
Zhou X, Douglas IJ, Shen R, Bate A. Signal detection for recently approved products: adapting and evaluating self-controlled case series method using a US claims and UK electronic medical records database. Drug Saf. 2018;41:523–36.
Dang T-T, Nguyen T-H, Ho T-B. Causality assessment of adverse drug reaction: controlling confounding induced by polypharmacy. Curr Pharm Des. 2019;25:1134–43.
Davazdahemami B, Delen D. Examining the effect of prescription sequence on developing adverse drug reactions: the case of renal failure in diabetic patients. Int J Med Inf. 2019;125:62–70.
Duan R, Boland MR, Moore JH, Chen Y. ODAL: a one-shot distributed algorithm to perform logistic regressions on electronic health records data from multiple clinical sites. Pac Symp Biocomput Pac Symp Biocomput. 2019;24:30–41.
Yu Y, Ruddy KJ, Wen A, Zong N, Tsuji S, Chen J, et al. Integrating electronic health record data into the ADEpedia-on-OHDSI platform for improved signal detection: a case study of immune-related adverse events. AMIA Jt Summits Transl Sci Proc AMIA Jt Summits Transl Sci. 2020;2020:710–9.
Yu Y, Nie X, Song Z, Xie Y, Zhang X, Du Z, et al. Signal detection of potentially drug-induced liver injury in children using electronic health records. Front Pediatr. 2020;8:171.
Zhang W, Peissig P, Kuang Z, Page D. Adverse drug reaction discovery from electronic health records with deep neural networks. Proc ACM Conf Health Inference Learn. 2020;2020:30–9.
Akimoto H, Nagashima T, Minagawa K, Hayakawa T, Takahashi Y, Asai S. Signal detection of potential hepatotoxic drugs: case–control study using both a spontaneous reporting system and electronic medical Records. Biol Pharm Bull. 2021;44:1514–23.
Nie X, Jia L, Peng X, Zhao H, Yu Y, Chen Z, et al. Detection of drug-induced thrombocytopenia signals in children using routine electronic medical records. Front Pharmacol. 2021. https://doi.org/10.3389/fphar.2021.756207.
Shin H, Lee S. An OMOP-CDM based pharmacovigilance data-processing pipeline (PDP) providing active surveillance for ADR signal detection from real-world data sources. BMC Med Inform Decis Mak. 2021;21:159.
Shin H, Cha J, Lee Y, Kim J-Y, Lee S. Real-world data-based adverse drug reactions detection from the Korea adverse event reporting system databases with electronic health records-based detection algorithm. Health Informatics J. 2021;27:14604582211033014.
Wu P, Nelson SD, Zhao J, Stone CA, Feng Q, Chen Q, et al. DDIWAS: High-throughput electronic health record-based screening of drug–drug interactions. J Am Med Inform Assoc JAMIA. 2021;28:1421–30.
Challa AP, Niu X, Garrison EA, Van Driest SL, Bastarache LM, Lippmann ES, et al. Medication history-wide association studies for pharmacovigilance of pregnant patients. Commun Med. 2022;2:115.
Kaas-Hansen BS, Placido D, Rodríguez CL, Thorsen-Meyer H-C, Gentile S, Nielsen AP, et al. Language-agnostic pharmacovigilant text mining to elicit side effects from clinical notes and hospital medication records. Basic Clin Pharmacol Toxicol. 2022;131:282–93.
Kundrot S, Warnick J, Erdman C, Robert K, Brown J. Demonstration of TreeScan techniques on a federated real-world data network. Drug Saf. 2022;45:1238–9.
Mower J, Bernstam E, Xu H, Myneni S, Subramanian D, Cohen T. Improving pharmacovigilance signal detection from clinical notes with locality sensitive neural concept embeddings. AMIA Jt Summits Transl Sci Proc. 2022;2022:349–58.
Nie X, Yu Y, Jia L, Zhao H, Chen Z, Zhang L, et al. Signal detection of pediatric drug-induced coagulopathy using routine electronic health records. Front Pharmacol. 2022;13: 935627.
Sauzet O, Cornelius V. Generalised weibull model-based approaches to detect non-constant hazard to signal adverse drug reactions in longitudinal data. Front Pharmacol. 2022. https://doi.org/10.3389/fphar.2022.889088.
Yu Y, Nie X, Zhao Y, Cao W, Xie Y, Peng X, et al. Detection of pediatric drug-induced kidney injury signals using a hospital electronic medical record database. Front Pharmacol. 2022. https://doi.org/10.3389/fphar.2022.957980.
Murphy RM, Klopotowska JE, de Keizer NF, Jager KJ, Leopold JH, Dongelmans DA, et al. Adverse drug event detection using natural language processing: a scoping review of supervised learning methods. PLoS ONE. 2023;18: e0279842.
Kulldorff M. TreeScan software for the tree-based scan statistic. Available at: https://www.treescan.org.
Malec SA, Wei P, Bernstam EV, Boyce RD, Cohen T. Using computable knowledge mined from the literature to elucidate confounders for EHR-based pharmacovigilance. J Biomed Inform. 2021;117: 103719.
Tatonetti NP, Ye PP, Daneshjou R, Altman RB. Data-driven prediction of drug effects and interactions. Sci Transl Med. 2012;4:125ra31.
Harpaz R, DuMouchel W, LePendu P, Bauer-Mehren A, Ryan P, Shah NH. Performance of pharmacovigilance signal-detection algorithms for the FDA adverse event reporting system. Clin Pharmacol Ther. 2013;93:539–46.
Kuhn M, Letunic I, Jensen LJ, Bork P. The SIDER database of drugs and side effects. Nucleic Acids Res. 2016;44:D1075-1079.
Dathe K, Schaefer C. The use of medication in pregnancy. Dtsch Arzteblatt Int. 2019;116:783–90.
Stock SJ, Norman JE. Medicines in pregnancy. F1000Research. 2019;8:911.
Braillon A, Bewley S. Prescribing in pregnancy shows the weaknesses in pharmacovigilance. BMJ. 2018;361: k2334.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This project was supported by Task Order 7540119F19002 under Master Agreement 75F40119D10037 from the US FDA. The FDA reviewed and approved this manuscript, however they had no role in data collection, management, or analysis. The views expressed represent those of the authors and do not necessarily represent the official views of the FDA.
Conflict of interest
Sharon E. Davis, Luke Zabotka, Rishi J. Desai, Shirley V. Wang, Judith C. Maro, Kevin Coughlin, José J. Hernández-Muñoz, Danijela Stojanovic, Nigam H. Shah, and Joshua C. Smith declare no competing interests.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Code availability
Not applicable.
Author contributions
SED and JCS designed the study and drafted the initial manuscript. SED, JCS, LZ, and KC contributed to the abstract screening and data extraction for the included studies. RJD, SVW JCM, JJH, DS, and NHS provided critical review of the included studies and interpretation of the results. All authors contributed to the final version of the manuscript and approved submission.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Davis, S.E., Zabotka, L., Desai, R.J. et al. Use of Electronic Health Record Data for Drug Safety Signal Identification: A Scoping Review. Drug Saf 46, 725–742 (2023). https://doi.org/10.1007/s40264-023-01325-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-023-01325-0