Abstract
Introduction
Adverse drug reactions (ADRs) contribute to morbidity, and serious ADRs may cause hospitalisation and death. This study characterises and quantifies ADR-related hospitalisations and subsequent in-hospital deaths, and estimates the spontaneous reporting rate to regulatory authorities in Switzerland, where healthcare professionals are legally obliged to report ADRs.
Methods
This retrospective cohort study from 2012 to 2019 analysed nationwide data from the Federal Statistical Office. ICD-10 coding rules identified ADR-related hospitalisations. To estimate the reporting rate, individual case safety reports (ICSRs) collected in the Swiss spontaneous reporting system during the same period were considered.
Results
Among 11,240,562 inpatients, 256,550 (2.3%) were admitted for ADRs, 132,320 (51.6%) were female, 120,405 (46.9%) were aged ≥ 65 (median of three comorbidities, interquartile range [IQR] 2–4), and 16,754 (6.5%) were children/teenagers (0 comorbidities, IQR 0-1). Frequent comorbidities were hypertension (89,938 [35.1%]), fluid/electrolyte disorders (54,447 [21.2%]), renal failure (45,866 [17.9%]), cardiac arrhythmias (37,906 [14.8%]), and depression (35,759 [13.9%]). Physicians initiated 113,028 (44.1%) of hospital referrals, and patients/relatives 73,494 (28.6%). Frequently ADR-affected were the digestive system (48,219 [18.8%], e.g. noninfective gastroenteritis and colitis), the genitourinary system (39,727 [15.5%], e.g. acute renal failure), and the mental/behavioural state (39,578 [15.4%], e.g. opioid dependence). In-hospital mortality was 2.2% (5669). Since ICSRs indicated 14,109 hospitalisations and 700 in-hospital deaths, estimated reporting rates were 5% and 12%, respectively.
Conclusions
This 8-year observation in Switzerland revealed that 2.3%, or roughly 32,000 admissions per year, were caused by ADRs. The majority of ADR-related admissions were not reported to the regulatory authorities, despite legal obligations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Little is known about ADR-related hospital admissions, subsequent in-hospital deaths, and spontaneous reporting rates in Switzerland on a national level. |
Approximately 2.3% of admissions are caused by ADRs, and the related in-hospital mortality is 2.2%. The estimated reporting rate is 5% and 12%, respectively, which emphasises the need for improved ADR reporting in hospitals. |
1 Introduction
Adverse drug reactions (ADRs) are an important cause of morbidity and mortality, and ADRs increase direct and indirect healthcare system costs [1,2,3,4]. However, substantial proportions of ADRs are deemed preventable, highlighting the potential of strategies to prevent ADRs and improve outcomes [1, 5].
ADRs that lead to hospitalisation are considered serious [6, 7]. Some Swiss research groups investigated ADR-related hospitalisations: Wasserfallen et al. conducted a single-centre study in a medical emergency department [4]. Fattinger et al. [8] and Hardmeier et al. [9] used data collected in two internal medicine departments. These studies, published from 2000 to 2004, found in those settings that 2.9–7% of hospital admissions were caused by ADRs.
In Switzerland, healthcare professionals are obliged by the Swiss Therapeutic Products Act to report serious or unlabelled ADRs to Swissmedic, the Swiss Agency for Therapeutic Products, which is responsible for the authorisation and surveillance of therapeutic products [6, 7, 10]. Nevertheless, one recent Swiss single-centre study on patients discharged from hospital concluded that only 3% of ADR-related readmissions were reported [11].
So far, no study investigated ADR-related hospitalisations in Switzerland on a national level. The primary objective of this nationwide study was to characterise and quantify ADR-related hospitalisations and subsequent in-hospital deaths. The secondary objective was to estimate the rate of spontaneous reporting of these serious ADRs to the regulatory authorities.
2 Methods
2.1 Study Design, Setting, Period, and Data Sources
This retrospective observational study analysed routinely collected data of the national inpatient cohort, featuring anonymous data as provided by the Swiss Federal Statistical Office [12]. The study period started when hospital reimbursement based on diagnosis-related groups (DRG) was put into effect in January 2012, and ended in December 2019 shortly before the COVID-19 pandemic reached Switzerland.
The national inpatient cohort dataset includes demographic variables, administrative variables such as information on health insurance, and medical variables such as ICD-10 coded diagnoses (International Classification of Diseases, World Health Organization [WHO], Geneva, Switzerland) [13], and information on in-hospital mortality of all hospitalisations in any Swiss hospital.
The first ICD-10 coded variable is the primary diagnosis, the second is designated for ancillary information related to the primary diagnosis, and the following variables 3–51 provide space for up to 49 secondary diagnoses. All available variables in the dataset are explained elsewhere [14]. The ICD-10 codes used in Switzerland are derived from the catalogue maintained by the German Federal Institute for Drugs and Medical Devices [15].
To estimate the reporting rate of individual case safety reports (ICSRs) involving a hospital stay to the Swiss regulatory authorities, the database of the global reporting system, VigiBase, [16] was queried for hospitalisations and in-hospital deaths in Switzerland during the study period. VigiBase collects ICSRs from member states participating in the international drug monitoring programme by the WHO (Geneva, Switzerland). All ICSRs from Switzerland, as collected by Swissmedic, are forwarded to VigiBase.
This study used completely anonymous data and conformed with the local law and the ethical review and research policies. Our study adhered to REporting of studies Conducted using Observational Routinely collected health Data (RECORD) [17].
2.2 ADR Definition Applied
The WHO defines an ADR as follows: “An adverse reaction to a drug is one that is noxious, is unintended, and occurs at doses normally used in man” [18]. ICSRs based on that strict definition are the most common type of ICSRs spontaneously reported to the Swiss regulatory authorities. However, Swissmedic states, “Although misuse, dependency and addiction are not covered by the WHO definition of an adverse drug reaction, as they do not relate to normal posology, it is important to report such events as they might affect the safety profile of a drug” [7]. It is therefore expected that spontaneous reports on misuse, dependency and addiction are also sent to the regulatory authorities. In other words, Swissmedic receives a higher number of ICSRs than what could be received if only ADRs according to the WHO definition would be reported.
Coleman and Pontefract wrote “Since 2012, the [ADR] definition has included reactions occurring as a result of error, misuse or abuse, and to suspected reactions to medicines that are unlicensed or being used off-label in addition to the authorised use of a medicinal product in normal doses”, [19, 20] which is in line with the current European Medicines Agency’s (EMA) guideline on good pharmacovigilance practices, stating, “Adverse reactions may arise from use of the product within or outside the terms of the marketing authorisation or from occupational exposure [DIR 2001/83/EC Art 101(1)]. Use outside the marketing authorisation includes off-label use, overdose, misuse, abuse and medication errors” [21].
For these reasons, this work applied the EMA’s ADR definition: “A response to a medicinal product which is noxious and unintended” [21].
2.3 Patients Admitted for ADRs
Each record in the inpatient cohort dataset provided one variable for the ICD-10 coded primary diagnosis, which was the reason for admission, and in this study, represented the ADR.
According to the Swiss ICD-10 coding rules, [22] ADR-related hospitalisations are coded in two ways, and both were considered in identifying our population of interest:
-
(i)
Hospitalisations related to ADRs may have a primary diagnosis explicitly describing an ADR, such as “G24.0 Drug-induced dystonia”, “M87.1 Osteonecrosis due to drugs”, “D68.3 Haemorrhagic disorder due to circulating anticoagulants” or “D59.2 Drug-induced nonautoimmune haemolytic anaemia” [13, 22]. The code list applied had been published by Hohl et al. [23] but only codes rated as A.1 “Induced by medication”, V “Induced by vaccine (added)”, A.2 “Induced by medication or other causes”, B.1 “Poisoning by medication”, B.2 “Poisoning by or harmful use of medication or other causes”, C “ADE very likely” (ADE stands for adverse drug event), or D “ADE likely” were considered. One code specific for Germany and Switzerland, “D70.1 Drug-induced agranulocytosis and neutropenia” [15] was added to complement that code list. Additionally, hospitalisations due to poisoning or harmful use according to the Swiss coding rules were also considered [22]. Of note, hospitalisations related to ADRs were not exclusively defined by the primary diagnoses mentioned above. A strength of our dataset in combination with the Swiss coding rules was that the ADR could be described by an extensive number of further primary diagnoses, as explained in the following.
-
(ii)
The next variable adjacent to the primary diagnosis can feature ICD-10 coded ancillary information that specifies what triggered the primary diagnosis. Among ancillary information codes were considered “Y57.9 Drug or medicament, unspecified” and, although rarely found, “Y59.9 Vaccine or biological substance, unspecified” both of which indicated that the primary diagnosis of the patient was an ADR, according to the Swiss coding rules [13, 22]. In some instances, hospitals entered the codes Y57.9 and Y59.9 into the further next adjacent variable, that is, the first secondary diagnosis, which was likewise considered. Other, more specific codes potentially indicating ADR-related hospitalisations (e.g. “Y42.0 Glucocorticoids and synthetic analogues” as ancillary information, leading to a diabetes-related primary diagnosis) were present in the past, however, this coding practice was abandoned in 2009, before the start of our study period.
Both approaches used to identify ADR-related hospitalisations are directly based on the Swiss coding rules [22]. That strategy allows for the most accurate ADR identification, as intended.
2.4 Data Processing and Statistics
One author (PEB) had full access to the data. No data cleaning, imputation or linkage was performed. Structured Query Language (SQL) statements were used to process and prepare the data for statistical analyses.
The patients’ secondary diagnoses were used to assign Elixhauser comorbidities (congestive heart failure, cardiac arrhythmias, valvular disease, pulmonary circulation disorders, peripheral vascular disorders, hypertension uncomplicated, hypertension complicated, paralysis, other neurological disorders, chronic pulmonary disease, diabetes uncomplicated, diabetes complicated, hypothyroidism, renal failure, liver disease, peptic ulcer disease excluding bleeding, AIDS/HIV, lymphoma, metastatic cancer, solid tumour without metastasis, rheumatoid arthritis/collagen vascular disease, coagulopathy, obesity, weight loss, fluid and electrolyte disorders, blood loss anaemia, deficiency anaemia, alcohol abuse, drug abuse, psychoses, depression) as defined by Quan et al. [24]. While renal failure was part of the Elixhauser comorbidities, we additionally assigned the Charlson Comorbidity dementia [24], on the basis of work by Parameswaran Nair et al. [25] (all comorbidities and their definitions are listed in supplementary table ST1).
Categorical variables are presented as counts and percentages and continuous variables with non-normal distributions as medians and interquartile ranges (IQR). Chi-squared tests compare categorical variables between groups and Kruskal–Wallis tests continuous variables.
If tables present the age of the patients, they stratify the patients by age group, or they show the proportion of patients aged ≥ 65, on the basis of work by Oscanoa et al. [26].
The primary diagnosis is the reason for admission and represents the ADR. To characterise the most frequent ADRs, they are first grouped according to the predominant ICD-10 chapters [13]. For each chapter exact numbers are presented regarding hospitalisations, patients aged ≥ 65, female patients, and in-hospital deaths. Then, the most frequent ADRs per chapter are shown with detailed numbers. In this ADR table, the last section is not an ICD-10 chapter but presents the remaining most frequent ADRs that did not fit in the predominant chapters.
The comparison of ADR-related admissions and in-hospital deaths based on the national inpatient cohort dataset versus the ICSRs involving a hospital stay collected by Swissmedic are juxtaposed and presented per study year, with proportions reflecting an estimate of the spontaneous reporting rate.
Statistical analyses were performed with R, version 4.1.1 (R Foundation for Statistical Computing, Vienna, Austria).
3 Results
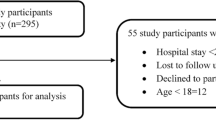
During the study period, 11,240,562 patients were discharged from Swiss hospitals (Fig. 1). Of these hospital stays, 10,984,012 (97.7%) were excluded:
-
(i)
a total of 93.7% (n = 10,531,247) were unrelated to medication issues,
-
(ii)
in 0.9% (n = 97,788) an adverse drug event (ADE) was considered as “unlikely” cause of admission, and
-
(iii)
in 3.2% (n = 354,977) an ADE could have been a “possible” cause of the admission [23].
Flow chart of considered versus excluded hospital admissions. All percentages are based on the total number of hospital stays. ADR, adverse drug reaction; ADE, adverse drug event. 1Admissions rated as U (“ADE unlikely”), 2admissions rated as E (“ADE possible”), 3admissions rated as A.1, V, A.2, B.1, B.2, C, or D, all according to the “Consensus Likelihood Rating” published by Hohl et al. [23]
A total of 256,550 patients (2.3%) were admitted for ADRs, and these admissions were analysed in detail.
3.1 Characteristics of Patients Admitted for ADRs
The patient characteristics are presented in Table 1. Almost half of ADR-related admissions (n = 120,405 [46.9%]) concerned patients aged 65 and older (median of three comorbidities, interquartile range [IQR] 2-4), whereas approximately 1 in 15 patients (n = 16,754 [6.5%]) was a child or a teenager (zero comorbidities, IQR 0–1). Female patients accounted for 51.6% (n = 132,320) of the admissions (cf. supplementary table ST2 for a sex-stratified version). Frequent comorbidities were hypertension (89,938 [35.1%]), fluid/electrolyte disorders (54,447 [21.2%]), renal failure (45,866 [17.9%]), cardiac arrhythmias (37,906 [14.8%]), and depression (35,759 [13.9%]). Overall, 54% of the patients had two or more comorbidities (cf. supplementary table ST3). Physicians initiated 44% of these hospital referrals, whereas in nearly 30% either the families or the patients themselves were responsible for the hospital referral.
3.2 Primary Diagnoses and Mortality
Table 2 presents the numbers of hospitalisations caused by ADRs which are represented by the primary diagnoses (cf. supplementary tables ST4 and ST5 for female and male subgroup versions, respectively). Frequently ADR-affected were the digestive system (48,219 [18.8%], e.g. noninfective gastroenteritis and colitis), the genitourinary system (39,727 [15.5%], e.g. acute renal failure), and the mental/behavioural state (39,578 [15.4%], e.g. opioid dependence). Of the patients admitted for ADRs, 2.2% (n = 5669) died during their hospital stay.
3.3 Estimation of Reporting Rate
A total of 14,109 ICSRs involving hospital stays were collected during the study period (Table 3). Thus, ca. one in 18 of such ADRs were reported to the Swiss regulatory authorities. Among them were 700 ICSRs with subsequent deaths. However, eight times more deaths were recorded in the inpatient dataset. Hence, the spontaneous reporting rate of ADR-related admissions and subsequent in-hospital deaths was estimated to be 5% and 12%, respectively. Had the applied ADR definition included admissions caused by “ADE possible” (cf. Fig. 1), reporting rates of 2% (14,109/611,527) and 3% (700/20,022) would have been calculated, respectively.
4 Discussion
In this retrospective analysis of 8 years of nationwide hospital data we found that 2.3% of admissions were ADR-related, averaging roughly 32,000 admissions per year in Switzerland. Almost half of admissions concerned patients aged 65 years and older, many of whom suffered from two or more comorbidities. Overall, 2.2% of the patients admitted for ADRs died during their stay.
4.1 Strengths
This analysis is based on a comprehensive nationwide dataset including all stays and all hospitals as a multicentre study. The Swiss ICD-10 coding rules allow for ADR coding either by means of explicit primary diagnosis codes or by adding indicative ICD-10 coded ancillary information. We believe that mostly unambiguous cases were accurately ADR coded after getting diagnosed by the physicians in charge of the patients. To our knowledge, this is the first study to investigate ADR-related hospitalisations in Switzerland on a national level. Further, our study appears to be the first to present evidence on the reporting rate for in-hospital mortality after ADR-related admissions independent of specific drugs or ADRs.
4.2 Limitations
This study has several limitations that need to be considered in interpreting our results. First, the dataset did not provide variables with drug information or provider notes that would have revealed the ADR-causing agents. This obvious downside could only have been circumvented if on-site chart reviews would have been performed, but the data were completely anonymous, hampering such approaches. Second, it was not possible to perform a causality assessment, since necessary data, for example, regarding the temporal relationship between drug and ADR, were lacking. Third, we were unable to distinguish between in-hospital mortality caused by ADRs and ADR-unrelated deaths. Forth, ICSRs involving hospital stays do not differentiate between ADRs causing and ADRs prolonging a hospitalisation, which may lead to overestimation of the reporting rate for ADR-related admissions. However, while it was feasible to identify ADR-related admissions in the inpatient dataset, it would be difficult – if not impossible – to reliably detect ADR-related prolongations of hospital stays.
4.3 Interpretation of Primary Results
The results of our analysis reveal that a significant number of hospital admissions in Switzerland is caused by ADRs. Leendertse et al. identified 95 studies reporting the prevalence of medication-related hospitalisations to be from 0.1% to 54% [27]. An ADR-focused review by Bouvy et al. [28] stated that the median prevalence of ADR-related hospital admissions was 3.5%, with a high variability ranging from 0.8% to 12.8% for studies performing “intensive chart review” to detect ADRs.
Patients aged 65 years and older accounted for almost half of ADR-related hospitalisations in the present study. Older populations are prone to ADRs due to increasing rates of polymedication and comorbidities such as renal failure [1, 29].
We found that most ADRs leading to admissions affected the digestive system. The study by Fattinger et al. [8] observed in two internal medicine departments that the involvement of the digestive system was the second leading cause for ADR-related admissions. A recent study by Kauppila et al. [30], who reviewed the charts of patients in the emergency department, also found that ADRs most frequently affected the digestive system.
With nearly 5% of the total number of ADR-related admissions, our single most frequent ADR was “Mental and behavioural disorders due to use of opioids: dependence syndrome”. This is especially concerning in light of a recent Swiss study by Hooijman and colleagues [31] that showed an increasing trend in strong opioids sold, particularly oxycodone, before and during our study period. However, whether our finding is related to prescribed or illicit opioids remains unknown.
In the present study of patients admitted for ADRs, the overall in-hospital mortality was 2.2%, which is comparable to other mortality data (e.g. Pirmohamed et al. [32] have reported 1.6% for Hospital A and 2.9% for Hospital B). A review of 43 studies presented a median mortality proportion of 1.7% for developed countries, [1] whereas a meta-analysis including 49 studies stated that 4.0% of patients admitted for ADRs died [33].
4.4 Interpretation of Reporting Rate
Various ADR definitions [34] changing over time, [35] may be partially responsible for the large variation of prevalences reported for ADR-related hospitalisations. We considered 2–7% to be a reasonable range, [35] and calculated a prevalence of 2.3%. On the basis of that proportion, we estimated the true reporting rate for ADR-related admissions and subsequent in-hospital deaths at 5% and 12%, respectively. In comparison, a systematic review by Hazell and Shakir presented eight studies using “intensive hospital-based monitoring” that had a median reporting rate of 4% [36]. Three of those studies focused on ADR-related admissions and stated reporting rates between 0.6% and 4.7% [37, 38]. In addition, a Swiss study on hospital readmissions due to ADRs within 30 days after discharge concluded that the reporting rate was 3% [11]. Taken together, the scarce published data on reporting rates of ADR-related admissions are roughly consistent with our results.
4.5 Generalisability
As we used comprehensive nationwide hospital data, we believe our study is representative for the whole of Switzerland. Concerning the generalisability of this work on an international level, different coding and reporting systems as well as differences in the handling of hospital admissions in other countries have to be taken into account. However, the generalisability of our findings is promoted by the application of the established ICD-10 code catalogue by Hohl et al. that provides a Consensus Likelihood Rating [23]. Additionally, most of our results are fairly in line with the data reported by the literature, as detailed above, which suggests plausibility.
5 Conclusions
In Switzerland, approximately 32,000 patients are admitted annually due to ADRs. Our study appears to be the first to present evidence on the reporting rate for in-hospital mortality after ADR-related admissions independent of specific drugs or ADRs. We estimate the rate of spontaneous reporting at 5% and 12% for ADR-related admissions and subsequent in-hospital deaths, respectively. A high proportion of serious ADRs, which are not reported to the responsible authorities or the marketing authorisation holders, is a well-known problem of spontaneous reporting systems worldwide. The real extent of this under-reporting remains somewhat unclear; however, our estimates add to the corpus of respective data. From a practical standpoint, in Switzerland and elsewhere, further measures to improve reporting are needed.
Future efforts concerning research should focus on reducing variability between studies and increasing the clarity and comparability of studies by improving definitions (e.g. ADR definition), by making inclusion and exclusion criteria clearer (e.g. whether all deaths or only ADR-related deaths were analysed), and by considering to change the standard of ICSRs involving hospital stays so that they differentiate between hospital admissions and prolongations.
References
Angamo MT, Chalmers L, Curtain CM, Bereznicki LRE. Adverse-drug-reaction-related hospitalisations in developed and developing countries: a review of prevalence and contributing factors. Drug Saf. 2016;39:847–57.
Sultana J, Cutroneo P, Trifirò G. Clinical and economic burden of adverse drug reactions. J Pharmacol Pharmacother. 2013;4:S73-77.
Rottenkolber D, Schmiedl S, Rottenkolber M, Farker K, Saljé K, Mueller S, et al. Adverse drug reactions in Germany: direct costs of internal medicine hospitalizations. Pharmacoepidemiol Drug Saf. 2011;20:626–34.
Wasserfallen J-B, Livio F, Buclin T, Tillet L, Yersin B, Biollaz J. Rate, type, and cost of adverse drug reactions in emergency department admissions. Eur J Intern Med. 2001;12:442–7.
Patel NS, Patel TK, Patel PB, Naik VN, Tripathi CB. Hospitalizations due to preventable adverse reactions-a systematic review. Eur J Clin Pharmacol. 2017;73:385–98.
Guideline IHT. Post-approval safety data management: definitions and standards for expedited reporting E2D. Eur Union Int Conf Harmon. 2003.
Swissmedic 2019 © Copyright. Reporting of suspected adverse drug reactions by patients [Internet]. https://www.swissmedic.ch/swissmedic/en/home/humanarzneimittel/marktueberwachung/pharmacovigilance/patienten-innen.html. Cited 5 June 2022.
Fattinger K, Roos M, Vergères P, Holenstein C, Kind B, Masche U, et al. Epidemiology of drug exposure and adverse drug reactions in two Swiss departments of internal medicine. Br J Clin Pharmacol. 2000;49:158–67.
Hardmeier B, Braunschweig S, Cavallaro M, Roos M, Pauli-Magnus C, Giger M, et al. Adverse drug events caused by medication errors in medical inpatients. Swiss Med Wkly. 2004;134:664–70.
SR 812.21—Federal Act of 15 December 2000 on Medicinal Products and Medical Devices (Therapeutic Products Act, TPA) [Internet]. https://www.fedlex.admin.ch/eli/cc/2001/422/en. Cited 5 June 2022.
Banholzer S, Dunkelmann L, Haschke M, Derungs A, Exadaktylos A, Krähenbühl S, et al. Retrospective analysis of adverse drug reactions leading to short-term emergency hospital readmission. Swiss Med Wkly. 2021;151: w20400.
Statistik B für. Medizinische Statistik der Krankenhäuser [Internet]. https://www.bfs.admin.ch/bfs/de/home/statistiken/gesundheit/erhebungen/ms.html. Cited 26 Nov 2021.
ICD-10 Version:2019 [Internet]. https://icd.who.int/browse10/2019/en. Cited 14 Nov 2021.
Statistik B für. Medizinische Statistik der Krankenhäuser - Variablen der Medizinischen Statistik. Spezifikationen gültig ab 1.1.2019 | Publikation [Internet]. Bundesamt Für Stat. 2018. https://www.bfs.admin.ch/asset/de/7066232. Cited 20 June 2022.
BfArM—ICD-10-GM Version 2022 [Internet]. https://www.dimdi.de/static/de/klassifikationen/icd/icd-10-gm/kode-suche/htmlgm2022/. Cited 14 June 2022.
Lindquist M. VigiBase, the WHO global ICSR database system: basic facts. Drug Inf J DIJ Drug Inf Assoc. 2008;42:409–19.
Benchimol EI, Smeeth L, Guttmann A, Harron K, Moher D, Petersen I, et al. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. PLoS Med. 2015;12: e1001885.
WHO Meeting on International Drug Monitoring: the Role of National Centres (1971: Geneva S, Organization WH. International drug monitoring : the role of national centres , report of a WHO meeting [held in Geneva from 20 to 25 September 1971] [Internet]. World Health Organization; 1972. https://apps.who.int/iris/handle/10665/40968.
Coleman JJ, Pontefract SK. Adverse drug reactions. Clin Med. 2016;16:481–5.
Directive EU. 84/EU of the European Parliament and of the Council of 15 December 2010 amending, as regards pharmacovigilance. Directive 2001/83/EC on the Community code relating to medicinal products …; 2010.
Agency EM. Guideline on good pharmacovigilance practices (GVP)-Annex I-Definitions (Rev 4). Heads Med Agencies. 2017;1–33.
Statistik B für. Instrumente zur medizinischen Kodierung [Internet]. https://www.bfs.admin.ch/bfs/de/home/statistiken/gesundheit/nomenklaturen/medkk/instrumente-medizinische-kodierung.html. Cited 15 June 2022.
Hohl CM, Karpov A, Reddekopp L, Doyle-Waters M, Stausberg J. ICD-10 codes used to identify adverse drug events in administrative data: a systematic review. J Am Med Inform Assoc JAMIA. 2014;21:547–57.
Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi J-C, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–9.
Parameswaran Nair N, Chalmers L, Bereznicki BJ, Curtain CM, Bereznicki LR. Repeat adverse drug reaction-related hospital admissions in elderly Australians: a retrospective study at the Royal Hobart Hospital. Drugs Aging. 2017;34:777–83.
Oscanoa TJ, Lizaraso F, Carvajal A. Hospital admissions due to adverse drug reactions in the elderly. A meta-analysis. Eur J Clin Pharmacol. 2017;73:759–70.
Leendertse AJ, Visser D, Egberts ACG, van den Bemt PMLA. The relationship between study characteristics and the prevalence of medication-related hospitalizations: a literature review and novel analysis. Drug Saf. 2010;33:233–44.
Bouvy JC, De Bruin ML, Koopmanschap MA. Epidemiology of adverse drug reactions in Europe: a review of recent observational studies. Drug Saf. 2015;38:437–53.
Kongkaew C, Noyce PR, Ashcroft DM. Hospital admissions associated with adverse drug reactions: a systematic review of prospective observational studies. Ann Pharmacother. 2008;42:1017–25.
Kauppila M, Backman JT, Niemi M, Lapatto-Reiniluoto O. Incidence, preventability, and causality of adverse drug reactions at a university hospital emergency department. Eur J Clin Pharmacol. 2021;77:643–50.
Hooijman MF, Martinez-De la Torre A, Weiler S, Burden AM. Opioid sales and opioid-related poisonings in Switzerland: a descriptive population-based time-series analysis. Lancet Reg Health Eur. 2022;20:100437.
Pirmohamed M, James S, Meakin S, Green C, Scott AK, Walley TJ, et al. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ. 2004;329:15–9.
Patel TK, Patel PB. Mortality among patients due to adverse drug reactions that lead to hospitalization: a meta-analysis. Eur J Clin Pharmacol. 2018;74:819–32.
Murdaugh L. Adverse drug reaction reporting. Competence Tools Health Syst Pharm. 2017;545–56.
Laville SM, Gras-Champel V, Moragny J, Metzger M, Jacquelinet C, Combe C, et al. Adverse drug reactions in patients with CKD. Clin J Am Soc Nephrol CJASN. 2020;15:1090–102.
Hazell L, Shakir SAW. Under-reporting of adverse drug reactions : a systematic review. Drug Saf. 2006;29:385–96.
Pouyanne P, Haramburu F, Imbs JL, Bégaud B. Admissions to hospital caused by adverse drug reactions: cross sectional incidence study. BMJ. 2000;320:1036.
Hallas J, Gram LF, Grodum E, Damsbo N, Brøsen K, Haghfelt T, et al. Drug related admissions to medical wards. Br J Clin Pharmacol. 1992;33:61–8.
Thakkar S, Slikker W, Yiannas F, Silva P, Blais B, Chng KR, et al. Artificial intelligence and real-world data for drug and food safety—a regulatory science perspective. Regul Toxicol Pharmacol. 2023;140: 105388.
Acknowledgements
We would like to thank Swissmedic and the Swiss Federal Statistical Office. We also acknowledge Dr. Corinne M. Hohl for responding to our email and answering our questions. Parts of preliminary data were shown on 4 October 2021, during a presentation at the 11th Global Summit on Regulatory Science (GSRS21 [39]) that took place in virtual format.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Open access funding provided by University of Zurich. This study was supported by the Swissmedic 4.0 initiative. The funding source played no role in the design and conduct of this study; the collection, management, analysis of the data or the interpretation of the results; or the review and approval of the manuscript.
Conflict of interest
The authors PEB, TS, and HD have no competing interests.
Ethics approval
The present investigation used completely anonymous data and conformed with the Swiss law and the ethical review and research policies.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Supplementary tables are available online with this publication. The data used in this study may be obtained from third parties but cannot be shared by the authors due to licensing issues and data protection requirements.
Code availability
The code of this study is available from the corresponding author upon reasonable request.
Author contributions
PEB conceived and designed the study, processed the data and performed the statistical analyses. All authors interpreted data. PEB drafted the work with TS and HD critically commenting on it. All authors approved the final submitted version of the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Beeler, P.E., Stammschulte, T. & Dressel, H. Hospitalisations Related to Adverse Drug Reactions in Switzerland in 2012–2019: Characteristics, In-Hospital Mortality, and Spontaneous Reporting Rate. Drug Saf 46, 753–763 (2023). https://doi.org/10.1007/s40264-023-01319-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-023-01319-y