Abstract
Over the course of the twentieth century, human life expectancy at birth rose in high-income nations by approximately 30 years, largely driven by advances in public health and medicine. Mortality reduction was observed initially at an early age and continued into middle and older ages. However, it was unclear whether this phenomenon and the resulting accelerated rise in life expectancy would continue into the twenty-first century. Here using demographic survivorship metrics from national vital statistics in the eight countries with the longest-lived populations (Australia, France, Italy, Japan, South Korea, Spain, Sweden and Switzerland) and in Hong Kong and the United States from 1990 to 2019, we explored recent trends in death rates and life expectancy. We found that, since 1990, improvements overall in life expectancy have decelerated. Our analysis also revealed that resistance to improvements in life expectancy increased while lifespan inequality declined and mortality compression occurred. Our analysis suggests that survival to age 100 years is unlikely to exceed 15% for females and 5% for males, altogether suggesting that, unless the processes of biological aging can be markedly slowed, radical human life extension is implausible in this century.
Similar content being viewed by others
Main
Before the middle of the nineteenth century, life expectancy at birth for humans languished at low levels by today’s standards—between 20 years and 50 years1. Improvements in survival were slow, punctuated often by episodic pandemics, plagues and contagions. Advances in public health and medicine in the early twentieth century spawned a longevity revolution characterized initially by large and rapid increases in life expectancy at birth (e(0)). e(0) increased at an accelerated rate, from an average of 1 year every one or two centuries for the previous 2,000 years to 3 years of life added per decade during the twentieth century (referred to a ‘radical life extension’). The variable pace of improvement in e(0) was influenced by geographic location, economic development and temporal factors2. This historic event began with reductions in early age mortality and continued later in the twentieth century with mortality improvements at middle and older ages3.
Given the profound influence of this longevity revolution on human society, one of the central questions in science, aging and public health today is: how much longer are humans capable of living? This is not a new question. Estimates of the highest theoretical life expectancy limits for national populations under optimal conditions is an exercise that dates back to the early twentieth century (for details, see Supplementary Note 1).
Accurately predicting future life expectancy trends holds important implications for societal, health and economic policies. In the United States, longstanding policy discussions have been held to address the potential consequences of how modulating biological aging could affect population demographics and related social institutions4,5,6. Accelerated population aging is already upon us; the absolute number of people reaching older ages continues to grow rapidly7; and the practical implications of such an intervention continue to deepen.
In 1990, it was hypothesized that humanity was approaching an upper limit to life expectancy (the limited lifespan hypothesis) in long-lived populations, as early gains from improved public health and medical care had largely been accomplished, leaving biological aging as the primary risk factor for disease and death; the rate of improvement in life expectancy was projected to decelerate in the twenty-first century; and e(0) for national populations would not likely exceed approximately 85 years (88 for females and 82 for males) unless an intervention in biological aging was discovered, tested for safety and efficacy and broadly distributed8. These conclusions were later supported by observed mortality dynamics in the United States, France and Japan from 1990 to 20009.
Claims were subsequently made that this limited lifespan view did not take into account ongoing advances in medicine and biology, that radical life extension (operationally defined here as a 0.3-year annual increase in period life expectancy at birth based on historical improvements in e(0) in long-lived populations in the twentieth century) had already begun10 or that it would soon occur due to the discovery and deployment of life-extending medical technologies and/or result from ongoing population-based improvements in behavioral risk factors11. It was further predicted that most newborns today will live to ages 100-plus years12 and that ‘… there is simply no convincing evidence (demographic, biological or otherwise) of a lower bound on death rates other than zero’13.
Three decades have now passed since the limited lifespan hypothesis was proposed. However, the debate continues between the limited lifespan and the radical lifespan extension hypotheses. In the present study, we used standard demographic survivorship metrics14,15 from the eight countries with the longest-lived populations and from the Special Administrative Region (SAR) of Hong Kong and the United States, from approximately 1990 to 2019, to address which hypothesis about human longevity is supported by these data. We then used this analysis to predict whether radical life extension is likely to occur again in this century. We used twentieth century increases in e(0) as a historical frame of reference.
In this analysis, we addressed the following questions. (1) Has radical life extension occurred at any time in the world’s longest-lived populations and in the United States from 1990 to 2019? (2) Is it plausible that most newborns today will live to age 100 years? (3) What is the rate of change in future mortality rates required to raise life expectancy at birth by 1 year? (4) What must human survival look like if radical life extension occurred again at any time in this century, and how plausible is this scenario? (5) Has the distribution of death in the longest-lived populations (measured by lifespan inequality) compressed in the last three decades or has it been expanding and shifting uniformly to later ages?
Results
Has radical life extension occurred at any time since 1990?
Observed annual age-specific and sex-specific death rates and period life expectancy at birth from 1990 to 2019 (the latter year was chosen to eliminate the effects of coronavirus disease 2019 (COVID-19)) in the eight countries with the longest-lived populations (that is, Australia, France, Italy, Japan, South Korea, Spain, Sweden and Switzerland) and in Hong Kong and the United States were chosen for this analysis (displayed here are annual changes in e(0)). Data were derived from the Human Mortality Database16.
Using these data, we found that the only populations to experience the required 0.3-year annual improvement or 3.0-year decennial improvement in life expectancy that operationally defines radical life extension (see Supplementary Note 2) were South Korea and Hong Kong17. In Hong Kong, this was largely due to economic prosperity and tobacco control but, even then, only from 1990 to 2000 (Fig. 1). In every population, including Hong Kong, the most recent decade of change in life expectancy is slower than it was in the last decade of the twentieth century. In every population but Hong Kong and South Korea, the annual rise in e(0) has decelerated to below 0.2 years annually.
Source: Human Mortality Database (2019 complete life tables by sex). Threshold annual improvement in life expectancy at birth of 0.3% is required to demonstrate that radical life extension has been or is occurring. The rate of improvement in life expectancy at birth has decelerated in every population except Hong Kong. The dashed line indicates the average annual change in e(0) associated with ‘radical life extension’.
The average increase in e(0) from 1990 to 2019 in Hong Kong and the eight countries with the longest-lived populations was 6.5 years. The United States is one of only a handful of countries documented to have experienced a lower life expectancy at birth at the end of any decade relative to the beginning of the same decade. This phenomenon also occurred in the early and middle part of the twentieth century but was historically caused only by extreme events (for example, the Spanish Flu and war-related deaths). In the United States, it was uniquely caused by a combination of increases in mortality at middle ages18 during the period 2010–2019 and then exacerbated by COVID-19 in 2020.
Predicting survival to age 100
Percentage survival to age 100 was derived from the l(x) (survivorship) columns of complete life tables for all nine countries and Hong Kong using period life tables in 2019 published by the Human Mortality Database.
In these populations, we found that the average probability of current birth cohorts surviving to age 100 is 5.1% for females and 1.8% for males (Fig. 2). The highest population-specific probability of surviving to 100 occurred in Hong Kong where 12.8% of females and 4.4% of males are expected to reach age 100 in their lifetime based on life tables from 2019. Across these eight countries with the longest-lived populations and in Hong Kong and the United States, we found no population that comes close to 50% survival to age 100 (Fig. 2).
Reduction in mortality required to raise e (0) by 1 year
The reduction in total mortality required to yield identical 1-year increases in e(0) is based on data from the Human Mortality Database for the populations evaluated here. The percentage reductions were derived by reducing total mortality at all ages, in each country, from as far back as 1750 (when the data existed) to 2019 until e(0) increased by 1 year.
In Hong Kong and all nine countries evaluated here, we found that the percentage reduction in total mortality required to raise e(0) by 1 year has actually increased relative to 1990 (Fig. 3).
As an illustration of the conditions present in most long-lived populations, we used period life tables for Japan in 2019 to calculate how much total mortality would have to decline, by gender, to experience a 1-year increase in e(0). If e(0) for females reaches 88 years in countries with long-lived populations, the magnitude of the reduction in death rates would be stark. Mortality from all causes at all ages required to raise life expectancy to 89 years for females would be 20.3%. For males, a rise from 82 years to 83 years would require a reduction in total mortality at every age of 9.5%.
The demographic rationale and the analytic derivation of lifespan metrics were derived from complete life tables published by the Human Mortality Database from 1950 to 2019. They include the log of e(0)—calculated as a function of life table entropy (H*) and lifespan inequality (Φ*) in accordance with equation (1), as shown in the Methods section. We estimated life table entropy using standard demographic techniques14,15,19,20.
The metrics that measure life table entropy (the dynamics of lifespan) are illustrated for the populations evaluated here from 1950 to 2019 in Figs. 4 and 5a,b.
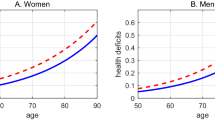
First, our analysis showed that the log of life expectancy at birth (log e(0)) increased continuously from 1950 through 2019 in all populations assessed (Fig. 4). This occurred at the same time period that life expectancy at birth decelerated in all countries with long-lived populations and maximum lifespan stagnated21.
Second, we found that the metric of life table entropy (H*) increased steadily from 1990 to 2019 (Fig. 5a). This likely occurred for a variety of reasons that are expected in humans: better health conditions, improved risk factors, medical technology, etc. However, H* increased, converged and stabilized at a common higher level in all long-lived populations, demonstrating that the force of life table entropy as a limiting force on rising life expectancy is greater today than it was in 1990.
Third, we evaluated lifespan inequality (also known as lifespan variation, Φ*)20. When Φ* declines, this represents empirical evidence that the mortality/survival distribution is compressing and the rise in life expectancy may be decelerating; when Φ* increases, this represents empirical evidence that the mortality/survival distribution is extending to later ages and the rise in life expectancy may be accelerating. As shown here (Fig. 5b), for the 69-year period from 1950 to 2019, Φ* declined uniformly and consistently in all long-lived populations, at the same time life expectancy was rising. This fact demonstrates that age at death has, in fact, been compressing into a shorter window of time at the outer reaches of human survival. It is theoretically possible for future mortality distributions to shift toward later ages, but the evidence is not supportive. Life expectancy has not increased since 1990 at a rate that defines radical life extension, nor is it likely to do so unless breakthroughs occur in retarding the rate of human aging.
Survival dynamics required for radical life extension
We generated a hypothetical survival distribution assuming radical life extension occurs again and e(0) rises to 110 years, using data from the Human Mortality Database for a population of Japanese females observed in 2019.
If e(0) were to hypothetically reach 110 years, death rates at all ages from all causes of death combined—up to age 150 years (for example, decades beyond the observed survival distribution for humans)—would need to be 88% lower than the observed death rate at age 109 in Japan in 2019. This level of mortality would require the complete cure or elimination of most major causes of death that exist today.
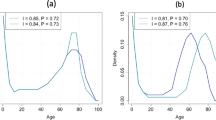
Our analyses demonstrate that a second wave of radical life extension, yielding a life expectancy at birth of 110 years at any time in the future, requires survival to age 100 by about 70% of females (Fig. 6, A). Survival to the age of 122.45 years—the maximum lifespan observed for humans as determined by the documented survival of Jeanne Calment, who died in 1997 (ref. 22)—would need to be achieved by over 24% of females to observe a life expectancy at birth of 110 years (Fig. 6, B). Radical life extension would also require about 6% of females to survive to ages 150-plus—28 years beyond the observed documented longest-lived human in history (Fig. 6, C).
Evidence for radical life extension requires a mortality distribution that is dramatically different from observed mortality distributions observed among the longest-lived human populations (denoted by A). A mortality distribution for a population experiencing radical life extension today requires just over one-fourth of the birth cohort surviving beyond the age of the maximum lifespan for the species (denoted by B). Approximately 6–7% of the birth cohort would need to survive to age 150 to achieve radical life extension (denoted by C).
Composite lowest age-specific and sex-specific death rates (2019)
A composite complete mortality schedule for humans (and related period life tables from ages 0–109 years for males and females) was compiled using the lowest age-specific and sex-specific mortality observed among all countries in the world based on period life tables published by the Human Mortality Database for 2019.
The life expectancy at birth associated with these composite mortality schedules as of 2019 is 88.68 years for females and 83.17 years for males. Higher potential life expectancies of 91.6 years for females and 86.1 years for males were estimated by Canudas-Romo et al.23 for the year 2039.
We found that the composite highest life expectancy mortality schedules in 2019 yield 13.9% survival to age 100 for females and 4.5% for males.
If death rates were zero from birth through age 50, the composite highest life expectancy at birth would be 89.7 for females and 84.7 for males. That is, reducing death rates to zero for the first 50 years of life adds 1.0 years to the composite most favorable life expectancy of females and 1.5 years to the composite most favorable life expectancy of males.
This composite set of death rates is nearly identical to a ‘best practice’ life expectancy used to justify forecasts of much higher life expectancies than those observed today24. Breaking through to death rates below those shown here at ages 0–65 to levels sufficient to have a meaningful influence on life expectancy at birth is viewed as implausible given observed trends in death rates at these ages in the past three decades in the longest-lived populations in the world.
Death rates beyond age 65 years can still plausibly go lower than the composite lowest death rates shown here25, but the magnitude of such declines would have to accelerate across time to generate an increase of 3 years per decade to reach the formal definition of radical life extension. This accelerated mortality improvement at older ages has not happened in the last three decades. Although a formally defined mortality floor cannot as yet be operationalized at older ages, there is evidence to suggest that such a floor does indeed exist, and it is not likely too far from the best practice composite death rates published here and by multiple other research teams that explore death rates and life expectancy for humans under optimal conditions (Supplementary Note 1).
Discussion
More than three decades have passed since predictions were made about the upper limits to human longevity. Evidence presented here based on observed mortality trends in the worldʼs eight longest-lived populations and in Hong Kong and the United States, and metrics of life table entropy, indicate that it has become progressively more difficult to increase life expectancy. The life table indicators are not only still operational; they are, in fact, a stronger limiting factor to rising e(0) today than they were in the late twentieth century. Although some countries have approached or reached the ‘limits’ to life expectancy that we hypothesized decades ago8, we found that, even in these countries, the rate of improvement in life expectancy has decelerated.
At ages 65 and older, the observed average rate of improvement in old-age mortality in the longest-lived populations evaluated here was 30.2% from 1990 to 2019. The impact of this level of mortality improvement, if experienced again over the next three decades, would yield only a 2.5-year increase in e(0). This is a fraction of the 3-year per decade (for example, 8.7-year increase from 1990 to 2019) gain in life expectancy predicted by those claiming that radical life extension was forthcoming or already here10. That is, old-age mortality has not been declining since 1990 at a pace that is even close to the rate of improvement required to achieve radical life extension in this century.
It is worth noting that radical life extension as defined here may occur in this century in some of today’s low-income or middle-income nations. This would be a product of experiencing the first longevity revolution in which death rates at younger and middle ages can still be reduced dramatically.
The importance of a mortality floor (referred to as a soft limit that can be breached by medicine and public health advances) shown here should be considered carefully by insurance companies and actuarial firms tasked with forecasting mortality improvement factors. These impact current carriers of life insurance and also the valuation of current and future insurance applicants’ policies26. If mortality improvement assumptions generated by organizations involved in survival forecasts yield age-specific and sex-specific death rates below the thresholds shown in Supplementary Table 1, justification for breaking through the current mortality floor should be required.
Moreover, as shown in Fig. 5a,b and equation (1), the logarithm of life expectancy can be decomposed into the sum of two terms where the first, life table entropy, is the dominant component in mortality improvements and the rise in life expectancy. When forecasting assumptions about life expectancy are based on a linear increase from past trends, then, by definition, the rate of improvement in death rates must accelerate, and metrics of life table entropy must improve accordingly. Our empirical measures of the rate of change in life expectancy and life table entropy in the current analysis demonstrate that this is not the case.
This means that extrapolating the metric of life expectancy from the past into the future is likely to yield overestimates of e(0) and survival, because this method of forecasting ignores the fundamental relation between life expectancy and the demographic metrics of life table entropy and lifespan inequality described here. This is why forecasts of longevity should be based on anticipated changes in death rates rather than linear projections of the metric of life expectancy.
There are limitations to this study, in particular related to the future. First, there can be no dispute that life expectancy improvements have decelerated since 1990. Our analysis clearly demonstrates that this finding runs counter to predictions that it was going to accelerate. Where uncertainty remains is how much more survival time can be manufactured with the disease model that now prevails (shown here to have a declining influence on life expectancy) and the far greater uncertainty associated with future improvements in survival that may result from the deployment of gerotherapeutics or other advances in medicine that cannot be conceived of today. Because radical lifespan extension brought forth by yet-to-be-developed medical advances cannot be empirically evaluated over short timeframes, a limitation here (and within the field of aging in general) is that it is difficult to justify any numerical estimate of their future influence on life expectancy.
Although limits to human life expectancy were discussed previously27, it is important to note that these limits do leave room for such advances in medicine (treating disease or targeting the underlying causes of aging28,29 and improved behavioral risk factors) that could further improve mortality at older ages (that is, these limits are not brick walls10 for longevity). Importantly, these limits should not be interpreted from an evolutionary perspective to mean that there is no longevity value in achieving grandparenthood30,31, that the post-reproductive period should necessarily be short32 or that the occurrence of chronic conditions of aging are driven by a force of selection to just beyond the upper edge of the reproductive window, in accordance with the antagonistic pleiotropy, mutation accumulation and disposable soma hypotheses33,34,35.
A naturally occurring life expectancy of a human population in the absence of any form of medical intervention throughout the course of life is unknown, but it would be expected to be far below the life expectancies observed in high-income nations today. As such, recent increases in e(0) are likely to be a result of the addition of what has been referred to as manufactured time—survival time brought forth by medical and public health interventions9.
The notion of a limit to life expectancy present today represents something akin to a glass mortality floor or a second soft limit to longevity that has become increasingly less sensitive to modifications through the treatment of diseases but that should be amenable to modification through changes in the rate of biological aging. The evidence presented here indicates that humans are approaching a second soft limit—using the very criteria set forth by those who have argued for the last three decades that either there is no limit to human longevity or, if there is one, it was not then in sight (Supplementary Note 3).
Forecasts about radical life extension in humans thought to be occurring now or projected to do so in the near term have already influenced the operations and financial structure of multiple industries36. Results presented here indicate that there is no evidence to support the suggestion that most newborns today will live to age 100 because this would first require accelerated reductions in death rates at older ages (the exact opposite of the deceleration that has occurred in the last three decades). Furthermore, even if the 30.2% improvements in mortality in the 65-and-older population observed to have occurred in high-income nations from 1990 to 2019 occurred again, only a small fractional increase in survival to age 100 would ensue. Changes in existing institutions that rely on lifespan estimation, such as retirement planning37 and life insurance pricing constructed with a 100-year lifespan assumption as its foundation, are likely to be overestimating survival by a wide margin for most people38.
It would be optimistic if 15% of females and 5% of males in any human birth cohort could live to age 100 in most countries in this century (even if any of the recently estimated composite best practice death rates are achieved under optimal conditions)—a limit that could theoretically be breached but only if gerotherapeutics are developed that slow biological aging. Even then, survival to age 100 for most people is not a certainty.
At a practical level, it is not feasible to test whether any current or future gerotherapeutic could extend human lifespan to over 100 years because of the time required to empirically verify life-extending properties of this magnitude. In fact, the efforts of the National Institute on Aging Interventions Testing Program (ITP) demonstrated that potential therapies have limited efficacy in safely extending mouse lifespan. Of 50 compounds so far tested, only 12 have increased lifespan (in either sex), none by more than 15% (ref. 39).
Increases in life expectancy at birth in countries with long-lived populations should not be confused with anticipated increases in the absolute number of people reaching older ages in this century40. The rising prevalence of older cohorts will accelerate across the globe in this century as a byproduct of larger birth cohorts born during the twentieth century41 moving up the age structure and as a result of improvements in survival at extreme old age due to medical advances. Large increases in the centenarian population across the globe, for example, can reliably be predicted to start in 2042, as this will mark the 100-year anniversary of the beginning of the post–World War II baby boom.
It is suggested here that humanity’s battle for a long life has largely been accomplished. This is not a pessimistic view of a longevity game over or that further mortality improvements at all ages (especially at older ages) are no longer possible or that healthspan can no longer be improved through risk factor modification or reductions in survival inequalities. Rather, it is a celebration of more than a century of public health and medicine successfully allowing humanity to gain the upper hand on the causes of death that have, thus far, limited human lifespan.
The evidence presented here indicates that the era of rapid increases in human life expectancy due to the first longevity revolution has ended (Supplementary Note 4). Given rapid advances now occurring in geroscience42, there is reason to be optimistic that a second longevity revolution is approaching in the form of modern efforts to slow biological aging, offering humanity a second chance at altering the course of human survival. However, until it becomes possible to modulate the biological rate of aging43 and fundamentally alter the primary factors that drive human health and longevity44, radical life extension in already long-lived national populations remains implausible in this century.
Methods
Demographic survivorship metrics
The link between improvements in age-specific death rates and life expectancy at birth (e(0)) is based on the following fundamental characterization, namely:
where H* denotes life table entropy, and Φ* is the lifespan inequality.
The analytic expression for these concepts was first developed more than a half century ago15; the lifespan inequality was later applied in an influential study of human longevity by gender45, followed more recently by a comprehensive review of these various metrics14.
The central parameters in the study of the age–survivorship function l(x) are as follows: the mean lifespan, the life table entropy and the lifespan inequality.
The mean lifespan is given by
The life expectancy e(0) can be expressed in terms of the ‘Entropy’ function H*, and an indicator of the distribution of ages at death (lifespan inequality) Φ*, with the following relationship:
Empirical studies20,46 of these life table metrics, as illustrated in Figs. 4 and 5a,b, are consistent with the following expected changes in the life table parameters during a time when a population’s health status is improving.
-
(i)
Life expectancy at birth, e(0), should increase.
-
(ii)
Life table entropy, H*, a positive correlate of the mean lifespan under ideal conditions, should increase.
-
(iii)
Lifespan inequality, Φ*, should decrease.
These three principles, and the fundamental linkages among the three metrics expressed in equation (1), indicate that radical changes in lifespan, as defined here, are contingent upon the rates at which the life table entropy and lifespan inequality change over time. We invoke these principles to empirically evaluate the plausibility of radical lifespan extension in long-lived populations.
Observed annual age-specific and sex-specific death rates and period life expectancy at birth from 1950/1990 to 2019 (the latter year was chosen to eliminate the effects of COVID-19) were used in this study. Data were derived from the Human Mortality Database that include the longest-lived populations in the world. The longest-lived populations chosen for this analysis were Australia, France, Hong Kong, Italy, Japan, South Korea, Spain, Sweden and Switzerland. If radical life extension has been occurring since 1990, it would most likely be observable in these populations. The United States was included because some scientists made specific predictions about radical life extension in this country.
Period life tables are preferred as the frame of reference because contemporary cohort life tables are accompanied by assumptions about future death rates (especially at older ages), and it is these very assumptions that are the subject of inquiry in this analysis.
Definition of radical life extension
The dramatic increase in life expectancy in humans occurred mostly during the twentieth century. In the populations evaluated here using data from the Human Mortality Database, the annual rate of increase in e(0) for males and females combined from 1900 to 2000 was about 0.33. Of note, there was considerable variation in the timing with which these improvements in life expectancy occurred across these populations in the twentieth century (for example, the Japanese, Hong Kong and South Korean populations concentrated their large increases in life expectancy only in the last quarter of the twentieth century). Thus, a 0.3-year annual rate of improvement in e(0) is considered a conservative operational definition of radical life extension.
Survival distribution with e (0) of 110 years
A hypothetical survival distribution assuming radical life extension occurs again and e(0) rises to 110 years was estimated using the following data and assumptions. (1) Data were drawn from a population of Japanese females observed in 2019 from the Human Mortality Database—intended to be representative of any long-lived population. (2) Period life expectancy at birth was assumed to rise to 110 years at an unspecified date in the future (which, in this case, was a 22.54-year increase from female life expectancy at birth observed in Japan in 2019). (3) Death rates at ages 110–150 years were assumed to remain constant at the level observed at age 109 in 2019 but then reduced proportionally in accordance with (4). (4) Death rates were reduced equally at all ages (including from ages 109–150) until e(0) reached 110. The estimated life table with radical life extension was closed at age 150.
Composite mortality schedule
A composite complete mortality schedule for humans (and related period life tables from ages 0–109 for males and females) was compiled using the lowest age-specific and sex-specific mortality observed among all populations in the world based on period life tables published by the Human Mortality Database for 2019. More recent years, although available in many cases, were not used as a way to avoid the negative influence of COVID-19 on the composite lowest mortality schedules.
Statistics and reproducibility
The results of the analyses presented here may be replicated using the data from the cited sources and the formulas presented above. The national vital statistics data for the nine countries and Hong Kong represent entire resident populations; data were not excluded from the analyses. The researchers were not blinded to the datasets during analysis. The population sizes for the populations evaluated ranged from a low of 7.3 million for Hong Kong to a high of 339 million for the United States.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data required to replicate the findings presented here include the Human Mortality Database (https://mortality.org/). All data supporting the findings of the study are available from the corresponding author upon reasonable request.
References
Riley, J. C. Rising Life Expectancy: A Global History (Cambridge Univ. Press, 2001).
Riley, J. C. Estimates of regional and global life expectancy, 1800–2001. Popul. Dev. Rev. 31, 537–543 (2005).
Eggleston, K. N. & Fuchs, V. R. The new demographic transition: most gains in life expectancy now realized late in life. J. Econ. Perspect. 26, 137–156 (2012).
Social Policy, Social Ethics, and the Aging Society (eds Neugarten, B. L. & Havighurst, R. J.) (Univ. of Chicago, 1976).
Public Meeting of the President's Council on Bioethics on December 12–13, 2002 (President’s Council on Bioethics, 2002); https://www.federalregister.gov/documents/2002/11/27/02-30045/public-meeting-of-the-presidents-council-on-bioethics-on-december-12-13-2002
The Fountain of Youth? The Quest for Aging Therapies (Congressional Committee on Science, Space, and Technology, 2022). https://www.congress.gov/event/117th-congress/house-event/115115?s=1&r=12
Olshansky, S. J., Carnes, B. A. & Cassel, C. K. The aging of the human species. Sci. Am. 268, 46–52 (1993).
Olshansky, S. J., Carnes, B. A. & Cassel, C. In search of Methuselah: estimating the upper limits to human longevity. Science 250, 634–640 (1990).
Olshansky, S. J., Carnes, B. A. & Désesquelles, A. Prospects for human longevity. Science 291, 1491–1492 (2001).
Oeppen, J. & Vaupel, J. W. Broken limits to life expectancy. Science 296, 1029–1031 (2002).
Kurzweil, R. & Grossman, T. Fantastic Voyage: Live Long Enough to Live Forever (Rodale Books, 2004).
Christensen, K., Doblhammer, G., Rau, R. & Vaupel, J. W. Ageing populations: the challenges ahead. Lancet 374, 1196–1208 (2009).
Wilmoth, J R. How long can we live? A review essay. Popul. Dev. Rev. 27, 791–800 (2001).
Fernandez, O. E. & Beltran-Sanchez, H. The entropy of the life table: a reappraisal. Theor. Popul. Biol. 104, 26–45 (2015).
Demetrius, L. Demographic parameters and natural selection. Proc. Natl Acad. Sci. USA 71, 4645–4647 (1974).
Human Mortality Database (MPIDR, accessed 1 July 2024); https://www.mortality.org/
Ni, M. Y. et al. Understanding Hong Kong’s path to becoming the world’s longest living population: a comparative study with long-living, high-income countries. Lancet Public Health 6, e919–e931 (2021).
Case, A. & Deaton, A. Rising morbidity and mortality in midlife among white non-Hispanic Americans in the 21st century. Proc. Natl Acad. Sci. USA 112, 15078–15083 (2015).
Demetrius, L. Natural selection, and age-structured population. Genetics 79, 535–544 (1975).
Demetrius, L. Adaptive value, entropy and survivorship curves. Nature 275, 213–214 (1978).
Dong, X., Milholland, B. & Vijg, J. Evidence for a limit to human lifespan. Nature 538, 257–259 (2016).
Robine, J. M., Allard, M., Herrmann, F. R. & Jeune, B. The real facts supporting Jeanne Calment as the oldest ever human. J. Gerontol. A Biol. Sci. Med. Sci. 74, S13–S20 (2019).
Canudas-Romo, V., Booth, H. & Bergeron-Boucher, M. P. Minimum death rates and maximum life expectancy: the role of concordant ages. N. Am. Actuar. J. 23, 322–334 (2019).
Torri, T. & Vaupel, J. W. Forecasting life expectancy in an international context. Int. J. Forecast. 28, 519–531 (2012).
Zuo, W., Jiang, S., Guo, Z., Feldman, M. W. & Tuljapurkar, S. Advancing front of old-age human survival. Proc. Natl Acad. Sci. USA 115, 11209–11214 (2018).
Olshansky, S. J., Willcox, B., Ashburn, K., Stukey, J. & Willcox, C. Personalized financial planning using applied genetics. Cold Spring Harb. Perspect. Med. 13, a041206 (2023).
Olshansky, S. J., Hayflick, L. & Carnes, B. A. Position statement on human aging. J. Gerontol. A Biol. Sci. Med. Sci. 57, B292–B297 (2002).
Warner, H. R. & Sierra, F. The longevity dividend: why invest in basic aging research? Can. J. Aging 28, 391–394 (2009).
Olshansky, S. J. & Carnes, B. A. Primary prevention with a capital P. Perspect. Biol. Med. 60, 478–496 (2017).
Hawkes, K. & Coxworth, J. E. Grandmothers and the evolution of human longevity: a review of findings and future directions. Evol. Anthropol. 22, 294–302 (2013).
Charnov, E. L. Life History Invariants: Some Explorations of Symmetry in Evolutionary Ecology (Oxford Univ. Press, 1993).
Vaupel, J. W. et al. Biodemographic trajectories of longevity. Science 280, 855–860 (1998).
Williams, G. C. Pleiotropy, natural selection, and the evolution of senescence. Evolution 11, 398–411 (1957).
Medawar, P. B. An Unsolved Problem of Biology (H. K. Lewis, 1952).
Kirkwood, T. B. L. Evolution of ageing. Nature 270, 301–304 (1977).
The New Map of Life (Stanford Center on Longevity, 2022); https://longevity.stanford.edu/wp-content/uploads/2022/04/new-map-of-life-full-report.pdf
Gratton, L. & Scott, A. J. The 100-Year Life: Living and Working in an Age of Longevity (Bloomsbury, 2016).
Gratton, L. & Scott, A. How work will change when most of us live to 100. Harvard Business Review (2016); https://hbr.org/2016/06/how-work-will-change-when-most-of-us-live-to-100
Harrison, D. E. et al. Astaxanthin and meclizine extend lifespan in UM-HET3 male mice; fisetin, SG1002 (hydrogen sulfide donor), dimethyl fumarate, mycophenolic acid, and 4-phenylbutyrate do not significantly affect lifespan in either sex at the doses and schedules used. Geroscience 46, 795–816 (2024).
Easterbrook, G. What happens when we all live to 100? The Atlantic (2014); https://www.theatlantic.com/magazine/archive/2014/10/what-happens-when-we-all-live-to-100/379338/
Bloom, D. E., Canning, D. & Lubet, A. Global population aging: facts, challenges, solutions & perspectives. Daedalus 144, 80–92 (2015).
Aging: Geroscience as the New Public Health Frontier 2nd edn (eds Kirkland, J. D. et al.) (Cold Spring Harbor Laboratory Press, 2024).
Duque, G. et al. Geroscience for the next chapter of medicine. J. Gerontol. A Biol. Sci. Med. Sci. 78, 791–792 (2023).
Olshansky, S. J. From lifespan to healthspan. JAMA 320, 1323–1324 (2018).
Keyfitz, N. Applied Mathematical Demography (Wiley, 1977).
Demetrius, L. & Ziehe, M. The measurement of Darwinian fitness in human populations. Proc. R. Soc. Lond. B 222, 33–50 (1984).
Acknowledgements
We would like to thank A. Sahasranaman for his contribution to the empirical analysis of life table metrics in an earlier version of the manuscript. Funding support for H.B.S. comes from grants by the National Institute on Aging (R01AG052030) and the National Institute of Child Health and Human Development (P2C-HD041022) to the California Center for Population Research at UCLA. Funding for B.J.W. comes from the National Institute on Aging (R01AG027060–Kuakini Hawaii Lifespan Study) and the National Institute for General Medical Sciences (P20GM125526–Kuakini HHP Center of Biomedical Research Excellence for Clinical and Translational Research on Aging). Funding for the broad dissemination of this study through open access was provided by the American Federation for Aging Research (AFAR).
Author information
Authors and Affiliations
Contributions
Conceptualization of the analysis was done by S.J.O. Methodology was developed by H.B.-S., S.J.O., B.J.W. and L.D. Visualization was created by H.B.-S., S.J.O. and L.D. Writing of the original draft was done by S.J.O., H.B.-S. and B.J.W. Reviewing and editing was completed by S.J.O., H.B.-S., B.J.W. and L.D.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Aging thanks Dmitri Jdanov and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Notes 1–4 and Table 1.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Olshansky, S.J., Willcox, B.J., Demetrius, L. et al. Implausibility of radical life extension in humans in the twenty-first century. Nat Aging 4, 1635–1642 (2024). https://doi.org/10.1038/s43587-024-00702-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43587-024-00702-3