Abstract
Background
Antibiotics are commonly used in both outpatient and inpatient settings and are responsible for the majority of adverse drug reaction (ADR) reports. We aimed to characterize spontaneously reported ADRs associated with antibiotics and assessing the preventability of these ADRs in a Vietnamese setting.
Materials and Methods
We conducted a retrospective descriptive study based on ADRs related to antibiotics spontaneously reported by healthcare workers to the National Pharmacovigilance Database of Vietnam (NPDV) between June 2018 and May 2019. The characteristics of included reports were descriptively analyzed. The preventability of reported ADRs was assessed using a standardized preventability scale. We identified the leading causes and described the characteristics associated with preventable ADRs (pADRs).
Results
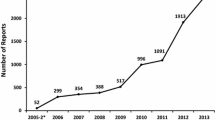
We included 6385 antibiotic-related reports from a total of 12,056 reports submitted to the NPDV during the study period. Beta-lactam antibiotics, mostly broad-spectrum with parenteral route, were suspected in the majority cases. The most commonly reported pADRs were allergic reactions, mostly classified under skin and subcutaneous tissue disorders. Of all included cases, 537 cases (8.4%) were deemed as associated with pADRs. Major causes of pADRs include potentially inappropriate prescribing (352/537, 65.5%) and re-administration of antibiotics causing prior allergy/allergies (99/537, 18.4%). The majority of pADRs involved the use of beta-lactam antibiotics with inappropriate indications.
Conclusion
ADRs related to antibiotic use represent more than half of ADRs spontaneously reported in Vietnam. Approximately one in every ten reported cases is associated with pADRs. The majority pADRs can be prevented through simple improvement in antibiotic prescribing practices.


Similar content being viewed by others
Data Availability
The datasets generated and/or analyzed in this study are not publicly available due to data protection law in Vietnam but might be available from the corresponding author on reasonable request.
References
Morimoto T, Gandhi T, Seger A, Hsieh T, Bates D. Adverse drug events and medication errors: detection and classification methods. BMJ Qual Saf. 2004;13(4):306–14.
European Medicines Agency. Good practice guide on recording, coding, reporting and assessment of medication errors. 2015. https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/good-practice-guide-recording-coding-reporting-assessment-medication-errors_en.pdf. Accessed 6 Mar 2022.
Krahenbuhl-Melcher A, Schlienger R, Lampert M, Haschke M, Drewe J, Krahenbuhl S. Drug-related problems in hospitals: a review of the recent literature. Drug Saf. 2007;30(5):379–407.
Howard RL, Avery AJ, Slavenburg S, Royal S, Pipe G, Lucassen P, et al. Which drugs cause preventable admissions to hospital? A systematic review. Br J Clin Pharmacol. 2007;63(2):136–47.
Villanueva P, Coffin SE, Mekasha A, McMullan B, Cotton MF, Bryant PA. Comparison of antimicrobial stewardship and infection prevention and control activities and resources between low-/middle-and high-income countries. Pediatr Infect Dis J. 2022;41(3):S3–9.
Hakkarainen KM, Sundell KA, Petzold M, Hägg S. Methods for assessing the preventability of adverse drug events. Drug Saf. 2012;35(2):105–26.
Olivier P, Boulbés O, Tubery M, Lauque D, Montastruc J-L, Lapeyre-Mestre M. Assessing the feasibility of using an adverse drug reaction preventability scale in clinical practice. Drug Saf. 2002;25(14):1035–44.
Imbs J, Pletan Y, Spriet A. Evaluation de la iatrogénèse médicamenteuse évitable: méthodologie. Thérapie (Paris). 1998;53(4):365–70.
Imbs J, Pouyanne P, Haramburu F, Welsch M, Decker N, Blayac J. Iatrogénie médicamenteuse: estimation de sa prévalence dans les hôpitaux publics français. Thérapie (Paris). 1999;54(1):21–7.
Sulis G, Daniels B, Kwan A, Gandra S, Daftary A, Das J, et al. Antibiotic overuse in the primary health care setting: a secondary data analysis of standardised patient studies from India, China and Kenya. BMJ Glob Health. 2020;5(9):e003393.
Nguyen KD, Vu DH, Nguyen HA, Dao VT, Montastruc JL, Bagheri H. Risk comparison of beta-lactam-induced anaphylaxis: therapeutic stratification analysis in a Vietnamese pharmacovigilance database. J Clin Pharm Ther. 2021;46(4):950–6.
Nguyen KD, Nguyen HA, Vu DH, Le TTL, Dang BV, Nguyen TN, et al. Drug-induced anaphylaxis in a Vietnamese pharmacovigilance database: trends and specific signals from a disproportionality analysis. Drug Saf. 2019;42(5):671–82.
Aagaard L, Strandell J, Melskens L, Petersen PS, Holme HE. Global patterns of adverse drug reactions over a decade: analyses of spontaneous reports to VigiBase. Drug Saf. 2012;35(12):1171–82.
Kanjanarat P, Winterstein AG, Johns TE, Hatton RC, Gonzalez-Rothi R, Segal R. Nature of preventable adverse drug events in hospitals: a literature review. Am J Health Syst Pharm. 2003;60(17):1750–9.
Karimian Z, Kheirandish M, Javidnikou N, Asghari G, Ahmadizar F, Dinarvand R. Medication errors associated with adverse drug reactions in Iran (2015–2017): a P-method approach. Int J Health Policy Manag. 2018;7(12):1090.
Nguyen KD, Nguyen PT, Nguyen HA, Roussin A, Montastruc JL, Bagheri H, et al. Overview of pharmacovigilance system in Vietnam: lessons learned in a resource-restricted Country. Drug Saf. 2018;41(2):151–9.
The Uppsala Monitoring Center. WHO adverse reaction terminology—WHO-ART. Sweden: TUM; 2012.
WHO Collaborating Centre for Drug Statistics Methodology. Structure and principles. 2005. https://www.whocc.no/atc/structure_and_principles/. Accessed 6 Mar 2022.
World Health Organization (WHO), Uppsala Monitoring Centre (UMC). The use of the WHO-UMC system for standardised case causality assessment. 2013. https://www.who.int/medicines/areas/quality_safety/safety_efficacy/WHOcausality_assessment.pdf. Accessed 6 Mar 2022.
US Department of Health and Human Services. Common terminology criteria for adverse events (CTCAE) Version 5.0. 2017. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf. Accessed 6 Mar 2022.
World Health Organization. ICD-10: international statistical classification of diseases and related health problems: tenth revision, 2nd ed. 2004. https://apps.who.int/iris/handle/10665/42980. Accessed 6 Mar 2022.
European Medicines Agency. ICH M5 EWG routes of administration controlled vocabulary. 2005. https://www.ema.europa.eu/en/documents/scientific-guideline/ich-m-5-ewg-routes-administration-controlled-vocabulary-step-5_en.pdf. Accessed 6 Mar 2022.
Vu TVD, Do TTN, Rydell U, Nilsson LE, Olson L, Larsson M, et al. Antimicrobial susceptibility testing and antibiotic consumption results from 16 hospitals in Viet Nam: the VINARES project 2012–2013. J Global Antimicrob Res. 2019;18:269–78.
Hoa NQ, Chuc NTK, Phuc HD, Larsson M, Eriksson B, Lundborg CS. Unnecessary antibiotic use for mild acute respiratory infections during 28-day follow-up of 823 children under five in rural Vietnam. Trans R Soc Trop Med Hyg. 2011;105(11):628–36.
Fair RJ, Tor Y. Antibiotics and bacterial resistance in the 21st century. Perspect Med Chem. 2014;6:25–64.
World Health Organization. Antimicrobial stewardship programmes in health-care facilities in low- and middle-income countries: a WHO practical toolkit. 2019. https://apps.who.int/iris/bitstream/handle/10665/329404/9789241515481-eng.pdf. Accessed 6 Mar 2022.
Van Nguyen K, Do NTT, Chandna A, Nguyen TV, Van Pham C, Doan PM, et al. Antibiotic use and resistance in emerging economies: a situation analysis for Viet Nam. BMC Public Health. 2013;13(1):1–10.
World Health Organization. Medication errors: technical series on safer primary care. 2016. https://apps.who.int/iris/bitstream/handle/10665/252274/9789241511643-eng.pdf. 6 Mar 2022.
Van Houten CB, Cohen A, Engelhard D, Hays JP, Karlsson R, Moore E, et al. Antibiotic misuse in respiratory tract infections in children and adults—a prospective, multicentre study (TAILORED Treatment). Eur J Clin Microbiol Infect Dis. 2019;38(3):505–14.
Cella M, Knibbe C, Danhof M, Della PO. What is the right dose for children? Br J Clin Pharmacol. 2010;70(4):597.
Iftikhar S, Sarwar MR, Saqib A, Sarfraz M. Causality and preventability assessment of adverse drug reactions and adverse drug events of antibiotics among hospitalized patients: a multicenter, cross-sectional study in Lahore, Pakistan. PLoS ONE. 2018;13(6):e0199456.
de Araújo BC, de Melo RC, de Bortoli MC, Bonfim JRDA, Toma TS. How to prevent or reduce prescribing errors: an evidence brief for policy. Front Pharmacol. 2019;10:1–7.
Nga DTT, Chuc NTK, Hoa NP, Hoa NQ, Nguyen NTT, Loan HT, et al. Antibiotic sales in rural and urban pharmacies in northern Vietnam: an observational study. BMC Pharmacol Toxicol. 2014;15(1):1–10.
Nguyen H-T, Nguyen T-D, van den Heuvel ER, Haaijer-Ruskamp FM, Taxis K. Medication errors in Vietnamese hospitals: prevalence, potential outcome and associated factors. PLoS ONE. 2015;10(9):e0138284.
Acknowledgements
We would like to thank Ms. Susan Luu and Ms. Ho Hong Ngoc from the WHO country office in Vietnam for constructive discussions to conceptualize study.
Funding
This study was funded by WHO (Project WPVNM1814680; Award No: 65763). The funders had no role in the design and conduct of the study; the analysis and interpretation of the data; the preparation; the review or approval of the manuscript. No other associations or activities might appear to have influenced the work that was presented.
Author information
Authors and Affiliations
Contributions
HDV and AHN conceptualized the research question and reviewed drafts of the manuscript. All authors co-developed the analysis plan. TNTN, HNT, NKT, LTN conducted the data analysis and contributed to the manuscript preparation. HNT and NTHT drafted the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare. We affirm that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of any organization or company.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tran, H.N., Nguyen, T.N.T., Tran, N.T.K. et al. Preventability of Adverse Drug Reactions Related to Antibiotics: An Assessment Based on Spontaneous Reporting System. Ther Innov Regul Sci 57, 1104–1112 (2023). https://doi.org/10.1007/s43441-023-00552-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43441-023-00552-y